��Ŀ����
��13�֣���������������ѭ��ѧԭ������������й����������̻ش����⡣
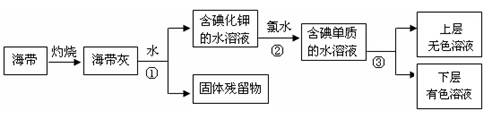
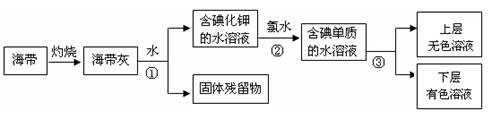
��1��ij��ѧ����С���Ժ���Ϊԭ�ϻ�������⣬���������ͼ��ʾ��
�����ٵ������� ��������ʹ�õ��Լ���д��ѧʽ�� �������� �з�����Ӧ�����ӷ���ʽΪ ��
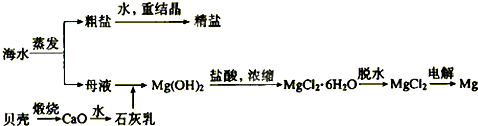
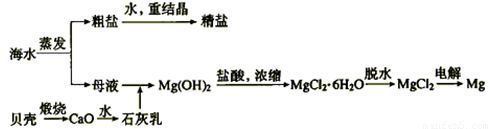
��2����ˮ���ۺ����ÿ����Ʊ������ƺ�þ������������ͼ��ʾ��
����������������Mg(OH)2���������ӷ���ʽΪ������ ��������Mg(OH)2���������ᷴӦ�����ӷ���ʽΪ ��
��ʵ�����ォ�����Ƴɾ��εĹ����У����ܽ⡢���ˡ�������������IJ����ж�Ҫ�õ����������ֱ�˵�������������ʹ�ò����������ã�
�ܽ�ʱ��____________������ʱ��____________������ʱ��______________��
�۹�ҵ�ϰѵ�ⱥ��ʳ��ˮ��Ϊ���ȼҵ����������ɵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ �� ��NaCl+�� ��H2O�� ��NaOH+H2��+�� ���� ����
![]()
��1������(1��)��CCl4(1��)��Cl2+2I-=I2+2Cl-(2��)
��2���� Mg2++Ca(OH)2= Mg(OH)2+Ca2+(2��)��Mg(OH)2 + 2H+ = Mg2+ + 2H2O��(2��)
�ڽ��裬�ӿ�����ܽ����ʣ�1�֣�����������Һ�ز������£�1�֣������裬ʹ��Һ�������ȣ���1�֣�
![]() ��2NaCl+2H2O 2NaOH+H2��+Cl2��(2��)
��2NaCl+2H2O 2NaOH+H2��+Cl2��(2��)
����:��



 �� ��NaOH+H2��+�� ���� ����
�� ��NaOH+H2��+�� ���� ����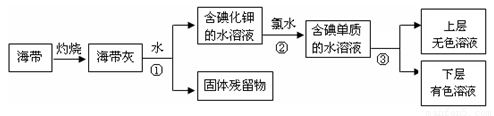
 �� ��NaOH+H2��+�� ���� ����
�� ��NaOH+H2��+�� ���� ����

 �� ��NaOH+H2��+�� ���� ����
�� ��NaOH+H2��+�� ���� ����