��Ŀ����
��10�֣�
 ��֪ij��ɫ������ĩ�к��� Al2 (SO4)3��Na2SO4��NH4HCO3��NH4Cl��KCl���������е����֡����������̽���������ɵ�ʵ��
��֪ij��ɫ������ĩ�к��� Al2 (SO4)3��Na2SO4��NH4HCO3��NH4Cl��KCl���������е����֡����������̽���������ɵ�ʵ��
��ѡ��������Ʒ���Լ����ձ����Թܡ�����������ͷ�ιܡ�ҩ�ס��ƾ��ơ�����ԹܼУ���ɫʯ����ֽ��1mol��L��1���ᡢ1mol��L��1���ᡢ1mol��L��1NaOH��Һ��Ba(NO3)2��Һ��AgNO3��Һ������ˮ��
 ����̽��
����̽��
ȡ������������ձ��У�������ˮ��ȫ�ܽ⣬�õ���ɫ����ҺA��ȡ����A���Թ��ڣ��μ�ϡ���ᣬ����ɫ���ݲ����������μ�ϡ��������Һ�в��ٲ������ݣ��õ���ɫ����ҺB��
 ��1������ʵ��֤�����û�����п϶����� �����ƣ����϶�������
����ѧʽ��
��1������ʵ��֤�����û�����п϶����� �����ƣ����϶�������
����ѧʽ��
 ��һ��̽��
��һ��̽��
��2��Ϊ�˽�һ��ȷ���û�Ϲ������ɣ���Ҫ����ʵ��1��ʵ��1��2������±���
|
ʵ����� |
Ԥ������ͽ��� |
|
ʵ��1��ȡ������ҺB���Թ��У��������� Ba(NO3)2��Һ�������ù۲졣 |
|
|
ʵ��2��
|
|
��1���û�����п϶�����̼����泥�2�֣����϶�������Al2(SO4)3��2�֣�
��2��
|
ʵ����� |
Ԥ������ͽ��� |
|
|
���������ɫ������˵��ԭ���������к���Na2SO4���û��������ΪNH4HCO3��Na2SO4��1�֣��� ���û�а�ɫ����������˵��ԭ���������в���Na2SO4������KCl��NH4Cl��1�֣��� |
|
���������ﲻ��Na2SO4��ȡ����ԭ�����������Թܣ���1�֣����Թ������ԹܼУ��û���ȼ�ƾ��ƣ���ּ��ȡ���1�֣� |
����Թܵײ��й��������˵��������к���KCl���û��������ΪNH4HCO3��KCl��1�֣�������Թܵײ����������˵��������к���NH4Cl���û��������ΪNH4HCO3��NH4Cl����1�֣� |
��������

 �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�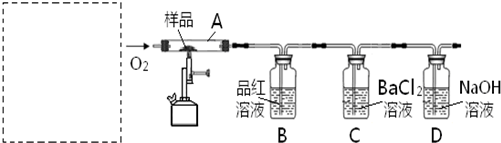
 CaO+CO2 �� 2CaO+2SO2+O2 =2CaSO4
CaO+CO2 �� 2CaO+2SO2+O2 =2CaSO4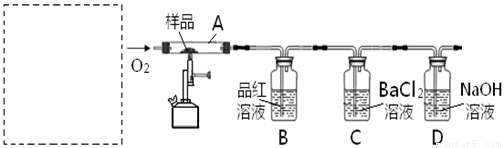
 CaO+CO2 ��2CaO+2SO2+O2=2CaSO4
CaO+CO2 ��2CaO+2SO2+O2=2CaSO4