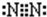��Ŀ����
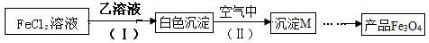
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ�����ת����ϵ��ͼ����ʾ��
��1����AΪ�����ڳ����������ʣ�B��C��Ϊ��AԪ�ص��Σ���B��Һ��pH��7��C��ҺpH��7�������ӷ���ʽ��ʾB��ҺpH��7��ԭ��______��
��2����A�ǵ���ɫ�����������D����ɫ���壻CΪ����ǿ����е����������Ӿ�Ϊ10�������ӣ�
��C��������ѧ����������______��
��д����ӦI�Ļ�ѧ����ʽ______��
�۽�һ����������Dͨ��2L��C����Һ�У���������Һ�б���μ���ϡ���� �������������������������������ʵ����Ĺ�ϵ��ͼ�ң�����������ܽ��HCl�Ļӷ�������ش�O����Һ���������ʵĻ�ѧʽΪ______��a����Һ�и�����Ũ���ɴ�С��˳����______��
�⣺��1����AΪ�����ڳ����������ʣ�B��C��Ϊ��AԪ�ص��Σ���B��Һ��pH��7��C��ҺpH��7��˵��AԪ�ؿ��γ�������ӣ���ӦΪAl����B����AlO2-��CӦ����Al3+������Һ�з���ˮ�ⷴӦ�����ӷ���ʽΪAlO2-+2H2O=Al��OH��3+OH-��
�ʴ�Ϊ��AlO2-+2H2O=Al��OH��3+OH-��
��2��A�ǵ���ɫ�������������ɫ���廯�����ǹ������ƺ��廯����������D����ɫ���壬A�ܺ�D��Ӧ������A�ǹ������ƣ�D�Ƕ�����̼���������ƺͶ�����̼��Ӧ����̼���ƺ�������B��E��Ӧ����C��C�к��е����������Ӿ�Ϊ10�������ӣ�C�ܺͶ�����̼��Ӧ����B������B��̼���ƣ�C���������ƣ�E��ǿ����������ơ�������������
��CΪNaOH��Ϊ���ӻ�����������Ӽ����ۼ����ʴ�Ϊ�����Ӽ����ۼ���
�ڷ�Ӧ��ΪNa2O2��CO2�ķ�Ӧ������ʽΪ2Na2O2+2CO2�T2Na2CO3+O2���ʴ�Ϊ��2Na2O2+2CO2�T2Na2CO3+O2��
�۷���ͼ�ң�3��5�ķ�ӦΪHCO3-+H+=CO2��+H2O����ʱ��������2mol��1��3�϶�����CO32-+H+=HCO3-���÷�ӦӦ����2molHCl������ʱ����3molHCl����϶�����NaOH��
��Ӧ��a����ҺΪNaHCO3����Һ�ʼ��ԣ���������Ũ�ȣ�Na+��Cl-��HCO3-��OH-��H+��CO32-��
�ʴ�Ϊ��NaOH��Na2CO3��Na+��Cl-��HCO3-��OH-��H+��CO32-��
��������1����AΪ�����ڳ����������ʣ�B��C��Ϊ��AԪ�ص��Σ���B��Һ��pH��7��C��ҺpH��7��˵��AԪ�ؿ��γ�������ӣ���ӦΪAl����B����AlO2-��CӦ����Al3+��
��2��A�ǵ���ɫ�������������ɫ���廯�����ǹ������ƺ��廯����������D����ɫ���壬A�ܺ�D��Ӧ������A�ǹ������ƣ�D�Ƕ�����̼���������ƺͶ�����̼��Ӧ����̼���ƺ�������B��E��Ӧ����C��C�к��е����������Ӿ�Ϊ10�������ӣ�C�ܺͶ�����̼��Ӧ����B������B��̼���ƣ�C���������ƣ�E��ǿ����������ơ���������������֤����ת����ϵ��
������������Ԫ�ػ�������ƶ�Ϊ���忼����Ԫ�ػ���������ʣ�ͬʱ����ѧ���������⡢����������������ȷ���ʵ������ǽⱾ��Ĺؼ����ѶȲ���
�ʴ�Ϊ��AlO2-+2H2O=Al��OH��3+OH-��
��2��A�ǵ���ɫ�������������ɫ���廯�����ǹ������ƺ��廯����������D����ɫ���壬A�ܺ�D��Ӧ������A�ǹ������ƣ�D�Ƕ�����̼���������ƺͶ�����̼��Ӧ����̼���ƺ�������B��E��Ӧ����C��C�к��е����������Ӿ�Ϊ10�������ӣ�C�ܺͶ�����̼��Ӧ����B������B��̼���ƣ�C���������ƣ�E��ǿ����������ơ�������������
��CΪNaOH��Ϊ���ӻ�����������Ӽ����ۼ����ʴ�Ϊ�����Ӽ����ۼ���
�ڷ�Ӧ��ΪNa2O2��CO2�ķ�Ӧ������ʽΪ2Na2O2+2CO2�T2Na2CO3+O2���ʴ�Ϊ��2Na2O2+2CO2�T2Na2CO3+O2��
�۷���ͼ�ң�3��5�ķ�ӦΪHCO3-+H+=CO2��+H2O����ʱ��������2mol��1��3�϶�����CO32-+H+=HCO3-���÷�ӦӦ����2molHCl������ʱ����3molHCl����϶�����NaOH��
��Ӧ��a����ҺΪNaHCO3����Һ�ʼ��ԣ���������Ũ�ȣ�Na+��Cl-��HCO3-��OH-��H+��CO32-��
�ʴ�Ϊ��NaOH��Na2CO3��Na+��Cl-��HCO3-��OH-��H+��CO32-��
��������1����AΪ�����ڳ����������ʣ�B��C��Ϊ��AԪ�ص��Σ���B��Һ��pH��7��C��ҺpH��7��˵��AԪ�ؿ��γ�������ӣ���ӦΪAl����B����AlO2-��CӦ����Al3+��
��2��A�ǵ���ɫ�������������ɫ���廯�����ǹ������ƺ��廯����������D����ɫ���壬A�ܺ�D��Ӧ������A�ǹ������ƣ�D�Ƕ�����̼���������ƺͶ�����̼��Ӧ����̼���ƺ�������B��E��Ӧ����C��C�к��е����������Ӿ�Ϊ10�������ӣ�C�ܺͶ�����̼��Ӧ����B������B��̼���ƣ�C���������ƣ�E��ǿ����������ơ���������������֤����ת����ϵ��
������������Ԫ�ػ�������ƶ�Ϊ���忼����Ԫ�ػ���������ʣ�ͬʱ����ѧ���������⡢����������������ȷ���ʵ������ǽⱾ��Ĺؼ����ѶȲ���

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
����ѧ--ѡ��3�����ʽṹ�����ʡ�
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��1��Gλ�� �� �����۵����Ų�ʽΪ ��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ ��
��4����֪BA5Ϊ���ӻ����д�������ʽ ��
��5��DE3����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ ��
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ��� ��
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
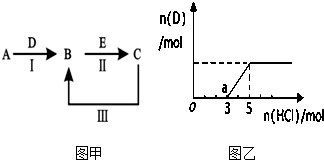
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�