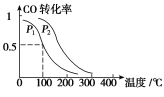
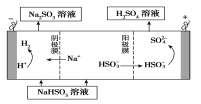
��Ŀ����
����Ŀ�������й�Na2O��Na2O2��������ȷ����
��Na2O��Na2O2���ܺ�ˮ��Ӧ���ɼ���Ƕ��Ǽ���������
��Na2O��CO2�������Ϸ�Ӧ����Na2CO3��Na2O2��CO2�����û���Ӧ����O2
��Na2O�ǵ���ɫ���ʣ�Na2O2�ǰ�ɫ����
��Na2O2��������������Na2O������������
��Na2O2��Na2O����ɫ��Ӧ��Ϊ��ɫ
A. ����ȷ B. �ڢۢܢ� C. �ڢۢ� D. �ܢ�
���𰸡�D
�����������������Na2O2���ᷴӦ�IJ�������κ�ˮ֮�⣬�����������ɣ����Բ��Ǽ���������������ų�A��Na2O2��CO2������Ӧ����O2�����û���Ӧ���������ų�B��C��ѡ��D��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
����Ŀ������ʵ�鲻�ܴﵽԤ��Ŀ�ĵ���
��� | ʵ������ | ʵ��Ŀ�� |
A | �� | �Ʊ� |
B | ���ˮ�еμӼ��� | �Ʊ� |
C | ��ij��Һ�м���ϡ | ������Һ���Ƿ��� |
D | ��ͬ�¶��£��ⶨŨ�ȷֱ�Ϊ0.1mol��L-1�� | �Ƚ� |
A. A B. B C. C D. D