��Ŀ����
(12��)�ú�������������ͭ��ȡ�Ȼ�ͭ���壨CuCl2��xH2O���������²�����

��֪����pHΪ4��5ʱ��Fe3+������ȫˮ���������Cu2+ȴ��ˮ�⡣
�� �������ܹ����з�����Ӧ�Ļ�ѧ����ʽ�У� ��
�� ������A��ѡ�� �����ţ���ͬ����
A.KMnO4 B. HNO3 C. Cl2
�� Ҫ�õ��ϴ��IJ�Ʒ���Լ�B��ѡ�� ��
A. NaOH B. CuO C.FeO
�� �Լ�B�������� ��
A. ʹCu2+��ȫ���� �� B. ʹFe3+��ȫ����
C. ������Һ��pH D. �����Һ��pH
�� ����Һ�����ᾧ�õ��Ȼ�ͭ�����ʵ�������� ��

��֪����pHΪ4��5ʱ��Fe3+������ȫˮ���������Cu2+ȴ��ˮ�⡣
�� �������ܹ����з�����Ӧ�Ļ�ѧ����ʽ�У� ��
�� ������A��ѡ�� �����ţ���ͬ����
A.KMnO4 B. HNO3 C. Cl2
�� Ҫ�õ��ϴ��IJ�Ʒ���Լ�B��ѡ�� ��
A. NaOH B. CuO C.FeO
�� �Լ�B�������� ��
A. ʹCu2+��ȫ���� �� B. ʹFe3+��ȫ����
C. ������Һ��pH D. �����Һ��pH
�� ����Һ�����ᾧ�õ��Ȼ�ͭ�����ʵ�������� ��
(12��)�� Fe + 2HCl = FeCl2 + H2�� CuO + 2HCl =CuCl2 + H2O
�� C �ۡ�B �� BD �� ����Һ�ڽϵ��¶��¼��������������壬ͬʱͨ���Ȼ����������ˮ�⡣����2�֣�
�� C �ۡ�B �� BD �� ����Һ�ڽϵ��¶��¼��������������壬ͬʱͨ���Ȼ����������ˮ�⡣����2�֣�
��

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ
 +4����ȡ����TiO2���������£�
+4����ȡ����TiO2���������£�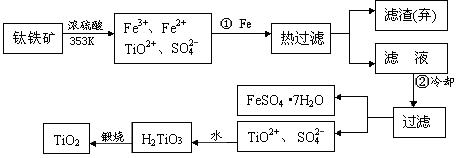
 TiCl4
TiCl4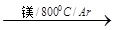 Ti
Ti 
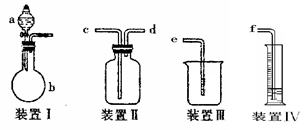
 �����
�����
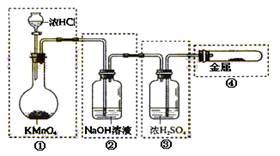
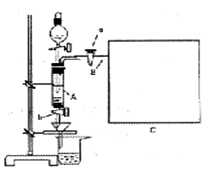
 ʵ��Ŀ�ĵ��ǣ� ��
ʵ��Ŀ�ĵ��ǣ� ��

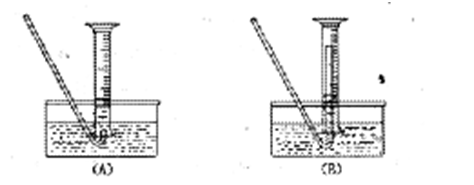
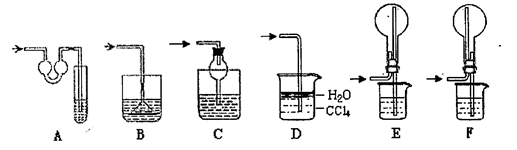
 ��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� ��
��һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ��� �� �����С�������� ��ζ��
�����С�������� ��ζ��