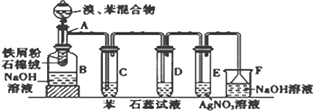
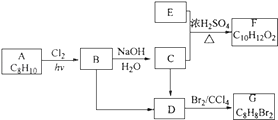
��Ŀ����
����Ŀ����2.32g Na2CO3��NaOH�Ĺ���������ȫ�ܽ���ˮ���Ƴ���Һ��Ȼ�������Һ����μ���1 mol/L�����ᣬ�����������������CO2�����(��״��)��ϵ����ͼ��ʾ������˵���д������

A. OA�η�����Ӧ�����ӷ���ʽΪ:H����OH��===H2O CO32-��H��===HCO3-
B. ������35mL����ʱ������CO2�����Ϊ224mL
C. A����Һ�е�����ΪNaC1��NaHCO3
D. �������NaOH������0.60g
���𰸡�D
�����������������A��OA�η�����Ӧ�����ӷ���ʽΪ��H++OH-�TH2O CO32-+H+�THCO3-����A��ȷ��B��AB�η�����Ӧ�����ӷ���ʽΪ��HCO3-+H+=H2O+CO2�������ݷ���ʽn(CO2)=(35-25)��10-3L��1mol/L=0.01mol�����Զ�����̼�����Ϊ��0.01mol��22.4L/mol=224mL����B��ȷ��C��OA�η�����Ӧ�����ӷ���ʽΪ��H++OH-�TH2O CO32-+H+�THCO3-������A�������ΪNaC1��NaHCO3����C��ȷ��D���ɷ�ӦHCO3-+H+=H2O+CO2����n(CO2)=n(Na2CO3)=(45-25)��10-3��1=0.02mol�������������Ƶ�����Ϊ2.32g-0.02��106g=0.2g����D����ѡD��

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�