��Ŀ����
����Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пհף�
��1������ЩԪ���У���ѧ��������õ��ǣ�
��2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��
��3������������������������Ԫ����
��4����ʾ�ٺ͢����ɵĻ�����ĵ���ʽ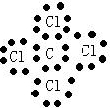
 ��
��
| ���� ���� |
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | �� | |||
| 4 | �� |
Ar
Ar
����Ԫ�ط��ţ�����2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��
HClO4
HClO4
���ܺ͢������������ˮ�������ǿ���ıȽϣ�KOH��NaOH
KOH��NaOH
������Ӧ��ѧʽ������3������������������������Ԫ����
Al
Al
����Ԫ�ط��ţ���д���������������������Ʒ�Ӧ�����ӷ���ʽAl2O3+2NaOH=2NaAlO2+H2O
Al2O3+2NaOH=2NaAlO2+H2O
����4����ʾ�ٺ͢����ɵĻ�����ĵ���ʽ


��������Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK��Ȼ����Ԫ�ص����ʼ���ѧ���������
����⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪS����ΪCl����ΪAr����ΪK��
��1������Ԫ��Ar����ã��ʴ�Ϊ��Ar��
��2������Ԫ����������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������K��Na���ܺ͢������������ˮ�������ǿ��ΪKOH��NaOH��
�ʴ�Ϊ��HClO4��KOH��NaOH��
��3������������������������Ԫ����Al����������ΪNaOH��Ӧ�����ӷ�ӦΪAl2O3+2NaOH=2NaAlO2+H2O��
�ʴ�Ϊ��Al��Al2O3+2NaOH=2NaAlO2+H2O��
��4���ٺ͢����ɵĻ�����ΪCCl4�������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��1������Ԫ��Ar����ã��ʴ�Ϊ��Ar��
��2������Ԫ����������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��������K��Na���ܺ͢������������ˮ�������ǿ��ΪKOH��NaOH��
�ʴ�Ϊ��HClO4��KOH��NaOH��
��3������������������������Ԫ����Al����������ΪNaOH��Ӧ�����ӷ�ӦΪAl2O3+2NaOH=2NaAlO2+H2O��
�ʴ�Ϊ��Al��Al2O3+2NaOH=2NaAlO2+H2O��
��4���ٺ͢����ɵĻ�����ΪCCl4�������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�����������⿼��λ�á��ṹ�����ʵĹ�ϵ����ϤԪ�������ڱ��е�λ���ƶ�Ԫ���ǽ��Ĺؼ�������Ԫ�ء�������֪ʶ�Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�±�ΪԪ�����ڱ���һ���������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1����д���ڵ�Ԫ�ط���
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�
| ������ | IA | |||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | |||
��2����д���۵����������ĵ���ʽ ��
��3���ȽϢݡ��ޡ����ԭ�Ӱ뾶�ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��
��4���ȽϢۡ��ܡ������ۺ������������ǿ������˳���ǣ�����Ļ�ѧʽ��ʾ�� ��
��5��д����Ԫ�آ�-��������������Ӧˮ�������ǿ������ǿ������֮��Ļ�ѧ��Ӧ����ʽ
��6���ߢ���Ԫ����Ƚϣ������Խ�ǿ���� �������ƣ���������֤�ý��۵�ʵ���� �����ţ���
��a�����ڿ����з����Ѿõ�������Ԫ�صĿ�״���ʷֱ������ˮ��
��b������״����С��ͬ��������Ԫ�صĵ��ʷֱ��ͬŨ�ȵ����ᷴӦ
��c������״����С��ͬ������Ԫ�صĵ��ʷֱ����ˮ���ã��������̪��Һ
��d���Ƚ�������Ԫ�ص���̬�⻯����ȶ��ԣ�
