��Ŀ����
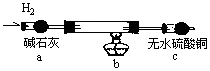
��(Sr)Ϊ�������ڢ�A��Ԫ�ء��ߴ���ˮ�Ȼ��Ⱦ���(SrCl2?6H2O)���кܸߵľ��ü�ֵ��61��ʱ���忪ʼʧȥ�ᾧˮ��100��ʱʧȥȫ���ᾧˮ���ù�ҵ̼���ȷ�ĩ(������Ba��Fe�Ļ�����)�Ʊ��ߴ���ˮ�Ȼ��ȵĹ�������ͼ��

��ش�
��1����������30%H2O2������ �������ӷ���ʽ��ʾ����
��2��������������ȷ�ĩ�������� ����ҵ����50��60���ȷ紵����ˮ�Ȼ��ȣ�ѡ����¶ȵ�ԭ���� ��
��3������ܽ��е�ʵ�����Ϊ �� ��������У�ϴ���Ȼ��Ⱦ������ѡ�� ��
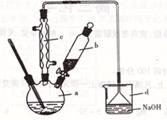
��4��ָ����ͼ������b ������ �� �ڳ���ʱʹ��ֽ��������©���ɰ�IJ����� ������ʹ��ֽ������©���ɰ��ϣ�
��5������ԭ��ҵ̼���ȷ�ĩ��̼���ȵ��������� ���м���ʽ���ɣ���

��ش�
��1����������30%H2O2������ �������ӷ���ʽ��ʾ����
��2��������������ȷ�ĩ�������� ����ҵ����50��60���ȷ紵����ˮ�Ȼ��ȣ�ѡ����¶ȵ�ԭ���� ��
��3������ܽ��е�ʵ�����Ϊ �� ��������У�ϴ���Ȼ��Ⱦ������ѡ�� ��
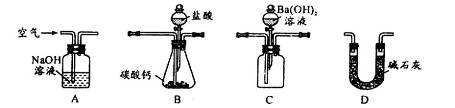
| A��ˮ | B������ | C������������Һ | D���Ȼ��ȱ�����Һ |

��5������ԭ��ҵ̼���ȷ�ĩ��̼���ȵ��������� ���м���ʽ���ɣ���
��1��H2O2+2Fe2++2H+=2Fe3++2H2O ��
��2������pH��ʹFe3+ת��Ϊ����������������ȥ���¶ȸ������ڳ�ȥʪ��ˮ�����¶ȸ���61��ʱ���Ȼ��Ⱦ����еĽᾧˮҲ��ʧȥ��
��3������Ũ�� ��ȴ�ᾧ D
��4������ƿ ������ˮ��ʪ��ֽ����ˮ��ͷ��
��5��
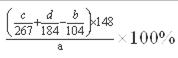
���������̼���������ᷴӦ�����Ȼ��ȡ�ˮ��������̼��̼���Ȳ�����ˮ���Ȼ���������ˮ����Ӧ���ӷ���ʽΪ��SrCO3+2H+=Sr2++H2O+CO2������Һ�д����Ȼ��ȣ����Լ��������������������ȣ� �������ᱵ���ܽ��С�������ȣ����Ի��һ��ת��Ϊ���ᱵ������˫��ˮ����ǿ�����ԣ������������£�˫��ˮ��Fe2+����ΪFe3+����������ԭΪH2O��(1) ��������30%H2O2�����ã��������������������������ӣ�(2) ������������ȷ�ĩ�������ǵ���pH��ʹFe3+ת��Ϊ����������������ȥ����ҵ����50��60���ȷ紵����ˮ�Ȼ��ȣ�ѡ����¶ȵ�ԭ�����¶ȸ������ڳ�ȥʪ��ˮ�����¶ȸ���61��ʱ���Ȼ��Ⱦ����еĽᾧˮҲ��ʧȥ��(3)����ܽ��е�ʵ�����Ϊ����Ũ������ȴ�ᾧ ��������У�ϴ���Ȼ��Ⱦ������ѡ���Ȼ��ȱ�����Һ��(4) ����b ����������ƿ�� �ڳ���ʱʹ��ֽ��������©���ɰ�IJ�����������ˮ��ʪ��ֽ����ˮ��ͷ������ʹ��ֽ������©���ɰ��ϣ�(5)������Ԫ���غ㣬ԭ��ҵ̼���ȷ�ĩ��̼���ȵ���������
 ��
�� 
��ϰ��ϵ�д�
�����Ŀ