��Ŀ����
����Ŀ����1�����и���ʵ��������ж���ȷ���� ����д��ĸ����
A������0.1mol/L CuSO4��Һ100 mL�������CuSO4��5H2O 1.6g
B������ƽ���������и���һ�Ű�ֽ�ɽ�NaOH����������̰�ֽ�ϳ���
C������Ͳ���Ծ�ȷ��ȡ25.03mLij��Һ��
D����Ҫ235 mL 0.9 mol/L NaCl��Һ��һ����250 mL����ƿ��������
E�������ƺ�һ�����ʵ���Ũ�ȵ���Һ��ע�����ˮϴ�����Լ�ƿ�У�Ũ�Ȳ���Ӱ�졣
��2��������ƿ�ϵı�ʶ���������еģ���д��ĸ��
A��ѹǿ B���¶� C���ݻ� D���ܶ� E���̶���
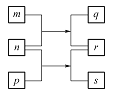
�����в����У�����ƿ�����߱��Ĺ����� (�����)��
A������һ�����ȷŨ�ȵı���Һ B������������Һ
C�����������ܽ�������� D����ȡһ�������Һ��
E���������������巢��װ��
��3����Ũ��������500mL2molL-1������Һʱ������IJ����������ձ�����Ͳ���������ͽ�ͷ�ι�֮�⣬����Ҫ �����������ƣ������������ƹ���ʾ��ͼ�У��д���IJ�������� ��

��4�� ��Ҫ������ƿ����250mL1.5molL-1Na2SO4��Һ,��õ���Һ�����Լ�ƿ����Ҫ���ϱ�ǩ�������������ͼ��ǩ�ϵ���������ȥ��
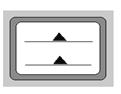
��5�����������ʹ������Һ�����ʵ���Ũ��ƫ�͵��� ������ţ���
A. ����ƿ������ˮϴ����, δ���ڱڸ������������
B. �ռ����ձ���պ���ȫ�ܽ�,��������Һת�Ƶ�����ƿ��
C. ����ʱ, ���ӿ̶���
D. ҡ�Ⱦ��ú���Һ��δ���̶���, ��������ˮ���̶���
E�����ܽ������������Һ�彦���ձ���
���𰸡�(1)D��2�֣�(2)��BCE��BCDE��2�֣�
(3)500mL����ƿ������1��2��4
(4) ��
�� ��1�֣�(5)CDE��2�֣�
��1�֣�(5)CDE��2�֣�
�������������������1��A������0.1mol/L CuSO4��Һ100mL�������CuSO45H2O 0.1mol/L��0.1L��250g/mol=2.5g��A����B��������ƽ����������ѭ�������룬Ӧ����Ʒ���������̳������������ƾ��и�ʴ�ԣ�Ӧ����С�ձ��г�����B����C����Ͳ��ȷ��Ϊ0.1mL��C����D����Ҫ235mL 0.9mol/L NaCl��Һ��ʵ����û��235mL����ƿ��Ӧѡ��250mL����ƿ��D��ȷ��E�������ƺ�һ�����ʵ���Ũ�ȵ���Һ��ע�����ˮϴ�����Լ�ƿ�У��൱����Һϡ�ͣ���ҺŨ��ƫ�ͣ�E����ѡD��
��2������ƿΪ����һ�����ʵ���Ũ����Һ��ר������������ƿ�ϱ��У��¶ȡ��ݻ����̶��ߣ���ѡBCE��
��3������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�����������Ͳ����ͷ�ιܡ����������ձ�������ƿ������500mL��Һ��Ҫ500mL����ƿ������IJ����������ձ�����Ͳ���������ͽ�ͷ�ι�֮�⣬����Ҫ500mL����ƿ��Ũ����ϡ��ʱӦ��Ũ���������ձ��ڻ���ע��ˮ�У�����������ʱ���������¶�Ӧ���ڿ̶���һ�£��������ƫ�����Դ���IJ���Ϊ��1��2��4��
��4���Լ�ƿ��ǩ��Ӧע��Ũ�Ⱥ�ҩƷ���ƣ����𰸣���
��5��A������ƿ������ˮϴ����δ���ڱڸ�����������ƣ�����Һ����������ʵ����ʵ���������Ӱ�죬��ҺŨ�Ȳ��䣬A��ѡ��B���ռ����ձ���պ���ȫ�ܽ⣬��������Һת�Ƶ�����ƿ�У���ȴ����Һ���ƫС����ҺŨ��ƫ��B��ѡ��C������ʱ�����ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�Cѡ��D��ҡ�Ⱦ��ú���Һ��δ���̶��ߣ���������ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�Dѡ��E�����ܽ������������Һ�彦���ձ��⣬���ڲ���������ģ����ʴ������ƫС����ҺŨ��ƫ�ͣ�Eѡ����ѡCDE��

 ��У����ϵ�д�
��У����ϵ�д�