��Ŀ����
��10�֣����ᷢ��������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ��ԭ�����е��Ļ��ϼ�Խ�͡�����������һ���������ۺ����۵Ļ������һ������ϡ��HNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4mol /LNaOH��Һ������NaOH��Һ�������mL����������������ʵ�����mol����ϵ��ͼ��ʾ����
��1��B��A�IJ�ֵ�� mol��
��2��B����ֵ�� mol��
��3��C����ֵ�� mL����Ҫ���м�����̣�
0.008 0.032 7
����

��ϰ��ϵ�д�
�����Ŀ
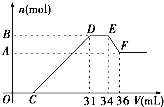 ���ᷢ��������ԭ��Ӧ��ʱ��һ������Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�ͣ�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ������˵���в���ȷ���ǣ�������
���ᷢ��������ԭ��Ӧ��ʱ��һ������Խϡ����Ӧ�Ļ�ԭ�����е��Ļ��ϼ�Խ�ͣ�����һ�������������Ͻ���һ����ϡHNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4mol/L NaOH��Һ������NaOH��Һ�������V����������������ʵ�����n����ϵ��ͼ��ʾ������˵���в���ȷ���ǣ�������| A���Ͻ������ᷴӦʱ������� | B���Ͻ����������ʵ���Ϊ0.008mol | C���Ͻ��н��������ʵ�����Ϊ0.032mol | D�������C���ֵ |
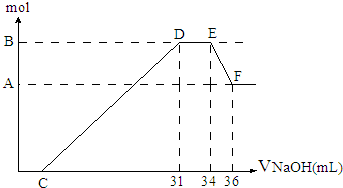
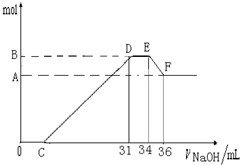 ���ᷢ��������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ��ԭ�����е��Ļ�����Խ�ͣ�����һ���������ۺ����۵Ļ������һ������ϡ��HNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1NaOH��Һ������NaOH��Һ�������mL����������������ʵ�����mol����ϵ��ͼ��ʾ��
���ᷢ��������ԭ��Ӧ��ʱ��һ������Ũ��Խϡ����Ӧ��ԭ�����е��Ļ�����Խ�ͣ�����һ���������ۺ����۵Ļ������һ������ϡ��HNO3��ַ�Ӧ����Ӧ���������κ�����ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1NaOH��Һ������NaOH��Һ�������mL����������������ʵ�����mol����ϵ��ͼ��ʾ��