��Ŀ����
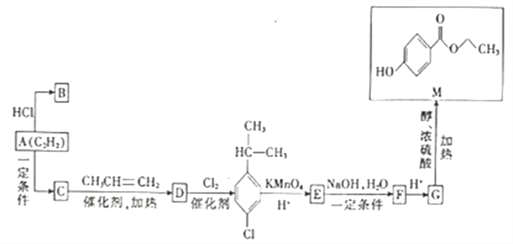
����Ŀ��ij����С�齫������Ĺ�ҵ������������Դ�����ã�����õĵ���������ȡ��84������Һ����֪��2H2S(g)+O2(g)= S2(s)+2H2O��1����H=-632kJ��mol-1����ͼΪ��С����Ƶ�ԭ��ͼ������˵����ȷ����

A. �缫a Ϊȼ�ϵ������
B. �缫b�Ϸ����ĵ缫��ӦΪ��O2+4e-+2H2O= 4OH-
C. ��·��ÿ����4mol ���ӣ�����ڲ��ͷ�����С��632 kJ
D. a��ÿ����32g��������e ���ռ�������22.4 L
���𰸡�C
��������A���缫a��H2S����ʧ���ӵ�������Ӧ����S2���缫aΪȼ�ϵ�صĸ�����A�����B���缫b��ͨ��O2���缫bΪ�������缫��ӦʽΪO2+4e-+4H+=2H2O��B�����C����·��ÿ����4mol������2molH2S��1molO2���뷴Ӧ���ͷ�����632kJ���˹��̽���ѧ��ת��Ϊ���ܺ����ܣ�����ڲ��ͷ�����С��632kJ��C����ȷ��D��a���缫��ӦʽΪ2H2S-4e-=S2+4H+��ʯī�缫c��缫a������ʯī�缫cΪ�������缫c�ĵ缫��ӦʽΪ2H2O+2e-=H2��+2OH-��������e�ռ���������ΪH2�����ݵ����غ㣬4n��S2��=2n��H2����n��H2��=2n��S2��=2![]() =1mol������H2�����¶Ⱥ�ѹǿδ֪�������㵼��e�ռ���H2�������D�����ѡC��
=1mol������H2�����¶Ⱥ�ѹǿδ֪�������㵼��e�ռ���H2�������D�����ѡC��

����Ŀ������ϩ����Ҫ�Ļ���ԭ�ϡ����ұ�(C6H5��CH2CH3)Ϊԭ�ϣ����ô�����ķ�����ȡ����ϩ(C6H5��CH=CH2)����Ӧ����ʽΪ��C6H5��CH2CH3(g)![]() C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
C6H5��CH=CH2(g)+H2(g) ��H=+117.6 kJ��mo1-1
�ش�����������
��1����֪��H2(g)+1/2O2(g)=H2O(l) ��H=-285.8 kJ��mo1-1
C6H5��CH2CH3(g)+21/2O2(g)=8CO2(g)+5H2O(l) ��H=-4607.1 kJ��mo1-1
��C6H5��CH=CH2(g)+10O2(g)= 8CO2(g)+4H2O(l) ��H=_____________��
��2����ҵ�ϣ��ں�ѹ�豸�н���������Ӧ��ȡ����ϩ�������ұ�������ͨ�����ˮ���������û�ѧƽ�����۽���ͨ�����ˮ������ԭ��_________________________________________��
��3����֪T���£���amol�ұ�����ͨ�뵽���ΪVL���ܱ������н���������Ӧ����Ӧʱ���������ڵ���ѹǿ�������±���
ʱ��t/min | 0 | 10 | 20 | 30 | 40 |
��ѹǿp/1000kPa | 1.0 | 1.3 | 1.45 | 1.5 | 1.5 |
���ɱ������ݼ���0~10 min��v(C6H5��CH2CH3)=________________�����ú�a��V ��ʽ�ӱ�ʾ��
���÷�Ӧƽ��ʱ�ұ���ת����Ϊ_________________________��
��4������ϩ���廯�ⷢ���ļӳɷ�Ӧ���������֣��䷴Ӧ����ʽ������
i.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CH2CH2Br (g)
C6H5��CH2CH2Br (g)
ii.C6H5��CH=CH2(g)+HBr(g)![]() C6H5��CHBrCH3(g)
C6H5��CHBrCH3(g)
600��ʱ����3L �����ܱ������г���1.2 mol C6H5��CH=CH2(g)��1.2 mol HBr(g)������Ӧ���ﵽƽ��ʱC6H5��CH2CH2Br (g)��C6H5��CHBrCH3(g)�����ʵ���(n)��ʱ��(t)�仯��������ͼ��ʾ��
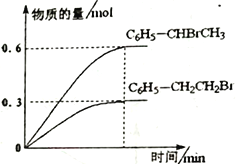
��600��ʱ����Ӧii �Ļ�ѧƽ�ⳣ��K ii=__________________��
����Ӧƽ��������������������䣬����������ٳ���1mol C6H5��CH2CH2Br (g)����Ӧii ��_________(��������������)�ƶ���
���ں��º��ݵ��ܱ������У�����ϩ���廯�ⷢ��i��ii�����ӳɷ�Ӧ���жϷ�Ӧ�Ѵﵽƽ��״̬����______��
B.C6H5��CH2CH2Br (g)������������C6H5��CHBrCH3 (g)�ֽ��������
C.��Ӧ����ѹǿ������ʱ��仯���仯
D.��������ƽ����Է����������ֲ���