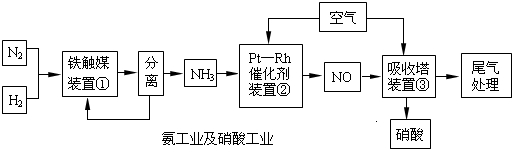
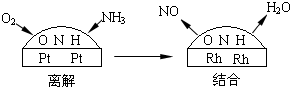
��Ŀ����
��ҵ�ϳɰ����Ʊ�����һ���������������������
��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO+H2O��g�� CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=________�������������
CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=________�������������
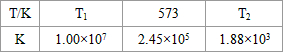
��2���ϳ����з�����ӦN2��g��+3H2��g�� 2NH3��g����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1_______300�棨�>������<����=������
2NH3��g����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1_______300�棨�>������<����=������
 CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=________�������������
CO2+H2��t��ʱ����1L�ܱ������г���0.2mol CO��0.3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol��L-1�����¶��´˷�Ӧ��ƽ�ⳣ��K=________���������������2���ϳ����з�����ӦN2��g��+3H2��g��
 2NH3��g����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1_______300�棨�>������<����=������
2NH3��g����H<0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1_______300�棨�>������<����=������ 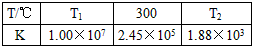
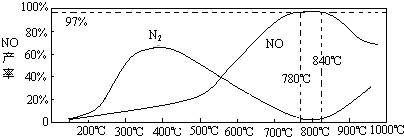
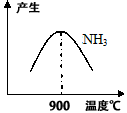
��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��_________________��
��4������������ͼ�У�����¯�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________��
��5�����᳧��β�����е�������������������ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g��= 4NO��g��+CO2��g��+2H2O��g����H= -574kJ��mol-1
CH4��g��+4NO��g��= 2N2��g��+CO2��g��+2H2O��g�� ��H= -1160kJ��mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��_________________��
��6�������ڴ�����ȼ�գ�����һ�ֵ��ʺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ______________����ѧ�����ô�ԭ������Ƴɰ���--����ȼ�ϵ�أ���ͨ�백���ĵ缫��________����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ___________________��
��5�����᳧��β�����е�������������������ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g��= 4NO��g��+CO2��g��+2H2O��g����H= -574kJ��mol-1
CH4��g��+4NO��g��= 2N2��g��+CO2��g��+2H2O��g�� ��H= -1160kJ��mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��_________________��
��6�������ڴ�����ȼ�գ�����һ�ֵ��ʺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ______________����ѧ�����ô�ԭ������Ƴɰ���--����ȼ�ϵ�أ���ͨ�백���ĵ缫��________����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ___________________��
��1��1
��2��<
��3���¶ȸ���900�棬ƽ�������ƶ�
��4��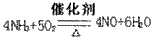
��5��
��6��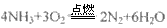 ��������
��������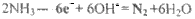
��2��<
��3���¶ȸ���900�棬ƽ�������ƶ�
��4��
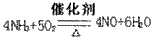
��5��

��6��
 ��������
��������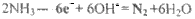

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ҵ�ϳɰ����Ʊ�����һ�������������������ͼ��
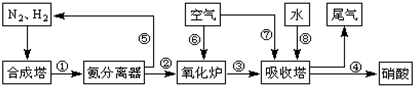
��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1 300�棨���������������=������
��3�������ڴ�����ȼ������һ�ֵ��ʺ�ˮ����ѧ�����ô�ԭ������Ƴɡ�����-������ȼ�ϵ�أ���ͨ�백���ĵ缫�� ����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ ��
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ ��
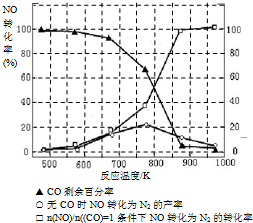
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ ����
=1�������£�Ӧ���Ƶ�����¶��� ���ң�

��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K=
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1
| T/�� | T1 | 300 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ
| n(NO) |
| n(CO) |

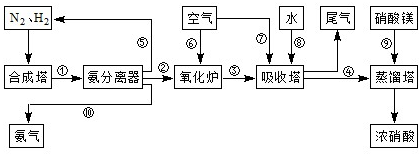
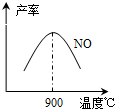 ��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��
��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��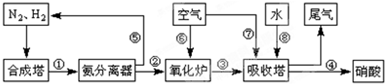
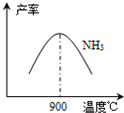 ��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��
��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��