��Ŀ����
��2011?��һģ����ҵ�ϳɰ����Ʊ�����һ��������������������£�
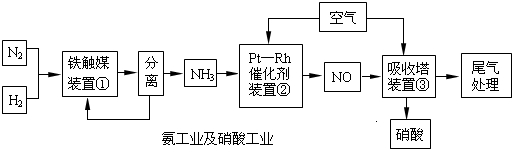
����������⣺
�ϳɰ�
��1��д��װ�â��з�����Ӧ�Ļ�ѧ����ʽ��
��2����֪��һ�����¶��½���װ�âٵĵ�������������Ӻϳ��������Ļ������ѹǿ֮��Ϊ5��4����ת����Ϊ
���ĽӴ�����ԭ��
��3����900��װ�â��з�Ӧ�У�?
4NH3��g��+5O2��g��?4NO��g��+6H2O��g������H=-905.5kJ?mol-1 K1=1��1053 ��900�棩
4NH3��g��+4O2��g��?4N2O��g��+6H2O��g������H=-1103kJ?mol-1 K2=1��1061 ��900�棩
4NH3��g��+3O2��g��?2N2��g��+6H2O��g������H=-1267kJ?mol-1 K3=1��1067 ��900�棩
�������з�Ӧ�⣬����һ����������ã�
4NH3��g��+6NO��g��?5N2��g��+6H2O��g������H=-1804kJ?mol-1�������ܷ�������һ�������ķֽ⣮
����Ȼ�ѧ����ʽ��2NO��g��?N2��g��+O2��g������H=
��4����-��Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ��ʾ��
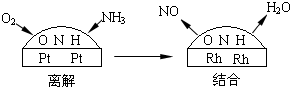
���ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ�ϣ�ʹ��NO��ˮ�����ڲ������Ѹ������������У���û��ʹ�ò�-��Ͻ�������������������Ҫ����
��5���¶ȶ�һ���������ʵ�Ӱ��
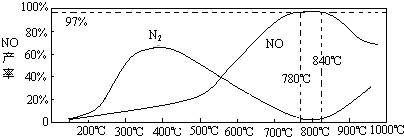
���¶ȴ���900��ʱ��NO�IJ����½���ԭ��
A���ٽ���һ�������ķֽ�
B���ٽ��˰��ķֽ�
C��ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
��6�����Ṥҵ��β������Na2CO3��Һ������β����NO��NO2��ȫ�������գ�д����Na2CO3��Һ���յķ�Ӧ����ʽ

����������⣺
�ϳɰ�
��1��д��װ�â��з�����Ӧ�Ļ�ѧ����ʽ��
N2+3H2
2NH3
| ���� |
| ���ȡ���ѹ |
N2+3H2
2NH3
��| ���� |
| ���ȡ���ѹ |
��2����֪��һ�����¶��½���װ�âٵĵ�������������Ӻϳ��������Ļ������ѹǿ֮��Ϊ5��4����ת����Ϊ
40%
40%
�����ĽӴ�����ԭ��
��3����900��װ�â��з�Ӧ�У�?
4NH3��g��+5O2��g��?4NO��g��+6H2O��g������H=-905.5kJ?mol-1 K1=1��1053 ��900�棩
4NH3��g��+4O2��g��?4N2O��g��+6H2O��g������H=-1103kJ?mol-1 K2=1��1061 ��900�棩
4NH3��g��+3O2��g��?2N2��g��+6H2O��g������H=-1267kJ?mol-1 K3=1��1067 ��900�棩
�������з�Ӧ�⣬����һ����������ã�
4NH3��g��+6NO��g��?5N2��g��+6H2O��g������H=-1804kJ?mol-1�������ܷ�������һ�������ķֽ⣮
����Ȼ�ѧ����ʽ��2NO��g��?N2��g��+O2��g������H=
-180.75 kJ?mol-1
-180.75 kJ?mol-1
����4����-��Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ��ʾ��

���ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ�ϣ�ʹ��NO��ˮ�����ڲ������Ѹ������������У���û��ʹ�ò�-��Ͻ�������������������Ҫ����
������ˮ����
������ˮ����
��˵�������Է�Ӧ��ѡ����
ѡ����
����5���¶ȶ�һ���������ʵ�Ӱ��

���¶ȴ���900��ʱ��NO�IJ����½���ԭ��
A��B
A��B
��ѡ����ţ�A���ٽ���һ�������ķֽ�
B���ٽ��˰��ķֽ�
C��ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
��6�����Ṥҵ��β������Na2CO3��Һ������β����NO��NO2��ȫ�������գ�д����Na2CO3��Һ���յķ�Ӧ����ʽ
NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2
NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2
����������1�������������ڴ��������ȼ�ѹ�����������ɰ�����
��2�����ݵ��������ϳɰ����Ļ�ѧ����ʽ���м��㣻
��3�����ݸ�˹���������㷴Ӧ���ʱ䣻
��4�������Է�Ӧ�Ĵ����þ���ѡ���ԣ�
��5��Ҫ���ͼ�������н�𣬶�������NO�ķ�Ӧ��һ��ʼ��Ӧû�дﵽƽ�⣬��NO�ﵽ��ߵ�ʱ���¶������ߣ�ƽ������Ű������ٵķ�����У���Ӧ��ת�����½���
��2�����ݵ��������ϳɰ����Ļ�ѧ����ʽ���м��㣻
��3�����ݸ�˹���������㷴Ӧ���ʱ䣻
��4�������Է�Ӧ�Ĵ����þ���ѡ���ԣ�
��5��Ҫ���ͼ�������н�𣬶�������NO�ķ�Ӧ��һ��ʼ��Ӧû�дﵽƽ�⣬��NO�ﵽ��ߵ�ʱ���¶������ߣ�ƽ������Ű������ٵķ�����У���Ӧ��ת�����½���
����⣺��1�������������ڴ��������ȼ�ѹ�����������ɰ����ķ�ӦΪ��N2+3H2
2NH3���ʴ�Ϊ��N2+3H2
2NH3��
��2���⣺�����ĵĵ���������x����N2 +3H2
2NH3
��ʼ���ʵ��� 1 3 0
�仯�����ʵ��� x 3x 2x
ƽ��ʱ�����ʵ��� 1-x 3-3x 2x
��������������Ӻϳ��������Ļ������ѹǿ֮��Ϊ5��4��������������������ʵ���֮����Ӻϳ��������Ļ����������ʵ���֮�͵ı�Ϊ5��4������
=
�����x=0.4mol�����Ե�����ת����Ϊ��
��100%=40%���ʴ�Ϊ��40%��
��3��������֪��Ӧ�ɵã���2NO��g��+3H2O��g��?2NH3��g��+
O2��g������H=
��905.5kJ?mol-1=452.75kJ?mol-1��
��2NH3��g��+
O2��g��?N2��g��+3H2O��g������H=-1267��
kJ?mol-1=-633.5kJ?mol-1��
��Ӧ2NO��g��?N2��g��+O2��g�����Կ����Ǣ�+�ڵĽ�������ԡ�H=-633.5kJ?mol-1+452.75kJ?mol-1=-180.75 kJ?mol-1���ʴ�Ϊ��-180.75 kJ?mol-1��
��4����û��ʹ�ò�-��Ͻ�������������������Ӧ��ʹ�õ������������ܷ�Ӧ��˵����������ѡ���ԣ��ʴ�Ϊ��������ˮ������ѡ���ԣ�
��5����ͼ���֪���¶Ƚϵ�ʱ���ɵ������¶ȸ���900��ʱ��NO�����½�����Ϊ��Ӧ4NH3+5O2?4NO+6H2O�Ƿ��ȷ�Ӧ���ﵽƽ�������ƽ��������У��ʲ��ʽ��ͣ�
�ʴ�Ϊ��A��B��
��6��β����NO��NO2��ȫ����̼�������գ��仯ѧ����ʽΪ��NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2��
�ʴ�Ϊ��NO+NO2+Na2CO3�T2NaNO2+CO2 ��2NO2+Na2CO3�TNaNO2+NaNO3+CO2��
| ���� |
| ���ȡ���ѹ |
| ���� |
| ���ȡ���ѹ |
��2���⣺�����ĵĵ���������x����N2 +3H2
| ���� |
| ���ȡ���ѹ |
��ʼ���ʵ��� 1 3 0
�仯�����ʵ��� x 3x 2x
ƽ��ʱ�����ʵ��� 1-x 3-3x 2x
��������������Ӻϳ��������Ļ������ѹǿ֮��Ϊ5��4��������������������ʵ���֮����Ӻϳ��������Ļ����������ʵ���֮�͵ı�Ϊ5��4������
| (1-x)+(3-3x)+2x |
| 4 |
| 4 |
| 5 |
| 0.4 |
| 1 |
��3��������֪��Ӧ�ɵã���2NO��g��+3H2O��g��?2NH3��g��+
| 5 |
| 2 |
| 1 |
| 2 |
��2NH3��g��+
| 3 |
| 2 |
| 1 |
| 2 |
��Ӧ2NO��g��?N2��g��+O2��g�����Կ����Ǣ�+�ڵĽ�������ԡ�H=-633.5kJ?mol-1+452.75kJ?mol-1=-180.75 kJ?mol-1���ʴ�Ϊ��-180.75 kJ?mol-1��
��4����û��ʹ�ò�-��Ͻ�������������������Ӧ��ʹ�õ������������ܷ�Ӧ��˵����������ѡ���ԣ��ʴ�Ϊ��������ˮ������ѡ���ԣ�
��5����ͼ���֪���¶Ƚϵ�ʱ���ɵ������¶ȸ���900��ʱ��NO�����½�����Ϊ��Ӧ4NH3+5O2?4NO+6H2O�Ƿ��ȷ�Ӧ���ﵽƽ�������ƽ��������У��ʲ��ʽ��ͣ�
�ʴ�Ϊ��A��B��
��6��β����NO��NO2��ȫ����̼�������գ��仯ѧ����ʽΪ��NO+NO2+Na2CO3�T2NaNO2+CO2��2NO2+Na2CO3�TNaNO2+NaNO3+CO2��
�ʴ�Ϊ��NO+NO2+Na2CO3�T2NaNO2+CO2 ��2NO2+Na2CO3�TNaNO2+NaNO3+CO2��
������������һ����ҵ�ϳɰ����ۺ���֪ʶ��Ŀ�����Ը�����ѧ֪ʶ���лش��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ