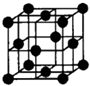
��Ŀ����
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ�ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl��Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ 36�����������������ش��������⣺������ʱҪ��Ԫ�ط��ű�ʾ��
��1��B�⻯����HCl��Ӧ���ɵĺ���BԪ�����ӵĿռ乹���� ��FԪ��ԭ�ӵ�����������Ϊ ����
��2��B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪ ��B3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬�������ӵ���ʽΪ �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���Ϊ
��3��A��B��C�ĵ�һ��������С�����˳��Ϊ
��4��E3+�ĺ�������Ų�ʽ�� ��ECl3�γɵ�����λ������ﻯѧʽΪ ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��6����F��+1��������ľ����ṹ����ͼ��FΪ ���ڡ����ס���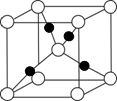
��1��B�⻯����HCl��Ӧ���ɵĺ���BԪ�����ӵĿռ乹����
��2��B3-���ӷֱ���AC2����B��C��ɵ���̬�����ﻥΪ�ȵ����壬��B��C��ɵĻ����ﻯѧʽΪ
��3��A��B��C�ĵ�һ��������С�����˳��Ϊ
��4��E3+�ĺ�������Ų�ʽ��
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��6����F��+1��������ľ����ṹ����ͼ��FΪ

������A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F��������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ���DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ�ԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d54s1����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ36������������=65-36=29����FΪCuԪ�أ��ݴ˽��
����⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F��������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ���DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�EԪ���ǵ�������Ԫ����δ�ɶԵ���������Ԫ�أ�ԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d54s1����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O��1mol�������������AgNO3��Һ��Ӧ����������3molAgCl������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��Fԭ�ӵ�һ�ֺ��ص�������Ϊ65��������Ϊ36������������=65-36=29����FΪCuԪ�أ�
��1��NԪ���⻯����HCl��Ӧ���ɵĺ���NԪ������ΪNH4+��������Nԭ�Ӽ۲���Ӷ���=4+
=4�������¶Ե��ӣ�����ռ乹�����������壻FΪCu��ԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d104s1��Ԫ��ԭ�ӵ�����������Ϊ1����
�ʴ�Ϊ���������壻1��
��2��N3-���ӷֱ���CO2����N��O��ɵ���̬�����ﻥΪ�ȵ����壬��N��O��ɵĻ����ﻯѧʽΪ N2O��
N3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬���ݼ۵���������ȣ���S��Cԭ�Ӵ���2��Nԭ�ӿ�֪��������ΪSCN-���ṹ��CO2���ƣ��������ӵ���ʽΪ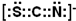 �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���ΪFe3+��
�����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���ΪFe3+��
�ʴ�Ϊ��N2O��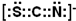 ��Fe3+��
��Fe3+��
��3��ͬ����������ҵ�һ�����ܳ��������ƣ���Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��4��Crԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d54s1��Crԭ��ʧȥ4s�ܼ�1�����ӡ�3d�ܼ�2�������γ�Cr3+��Cr3+�ĺ�������Ų�ʽ��1s22s22p63s23p63d3��������������֪��CrCl3�γɵ�����λ������ﻯѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d3��[Cr��NH3��4��H2O��2]Cl3��
��5��Mg��ϡ���ᷴӦ��NԪ�ر���ԭ����ͼۣ�Ӧ��������泥�����������þ��ˮ���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��6��Cu��+1��������ΪCu2O���ɾ����ṹ��֪�������к�ɫ����Ŀ=4����ɫ����Ŀ=1+8��
=2����ɫ�����ɫ����Ŀ֮��Ϊ2��1���ʺ�ɫ���ʾCu��
�ʴ�Ϊ���ڣ�
��1��NԪ���⻯����HCl��Ӧ���ɵĺ���NԪ������ΪNH4+��������Nԭ�Ӽ۲���Ӷ���=4+
| 5-1-1��4 |
| 2 |
�ʴ�Ϊ���������壻1��
��2��N3-���ӷֱ���CO2����N��O��ɵ���̬�����ﻥΪ�ȵ����壬��N��O��ɵĻ����ﻯѧʽΪ N2O��
N3-���ӻ����Ժ�һ�������ӻ�Ϊ�ȵ����壬���ݼ۵���������ȣ���S��Cԭ�Ӵ���2��Nԭ�ӿ�֪��������ΪSCN-���ṹ��CO2���ƣ��������ӵ���ʽΪ
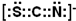 �����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���ΪFe3+��
�����������ӳ����ڼ����ճ������е�һ�ֽ��������ӣ�����������ӷ���ΪFe3+���ʴ�Ϊ��N2O��
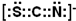 ��Fe3+��
��Fe3+����3��ͬ����������ҵ�һ�����ܳ��������ƣ���Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ��������С�����˳��ΪC��O��N��
�ʴ�Ϊ��C��O��N��
��4��Crԭ�Ӻ�������Ų�Ϊ��1s22s22p63s23p63d54s1��Crԭ��ʧȥ4s�ܼ�1�����ӡ�3d�ܼ�2�������γ�Cr3+��Cr3+�ĺ�������Ų�ʽ��1s22s22p63s23p63d3��������������֪��CrCl3�γɵ�����λ������ﻯѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��1s22s22p63s23p63d3��[Cr��NH3��4��H2O��2]Cl3��
��5��Mg��ϡ���ᷴӦ��NԪ�ر���ԭ����ͼۣ�Ӧ��������泥�����������þ��ˮ���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��6��Cu��+1��������ΪCu2O���ɾ����ṹ��֪�������к�ɫ����Ŀ=4����ɫ����Ŀ=1+8��
| 1 |
| 8 |
�ʴ�Ϊ���ڣ�
���������⿼�����ʽṹ�����ʣ���Ŀ�ۺ��Խϴ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ������ܡ�����ʽ���������Ų����������ӽṹ���ȵ����塢������ԭ��Ӧ�����������֪ʶ�㣬�Ѷ��еȣ��Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�

��ϰ��ϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

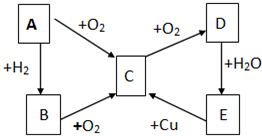 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�