��Ŀ����
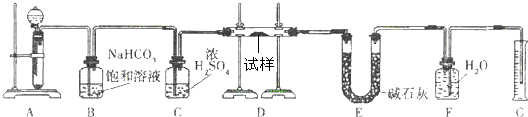
����һ��������Na2O���ʵ�Na2O2�����������ͼ��ѡ���ʵ���ʵ��װ�ã����һ�����ʵ�飬�ⶨNa2O2�����Ĵ��ȣ��ɹ�ѡ�õķ�Ӧ��ֻ��CaCO3���壬6Ħ��/�����������ˮ��������д���пհף�
��1��д��ʵ����Na2O2��Na2O�ֱ�����Ӧ�Ļ�ѧ����ʽ��______
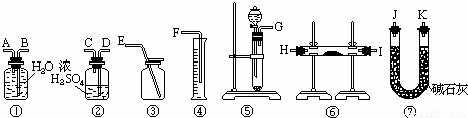
��2��Ӧѡ�õ�װ���ǣ�ֻҪ��д��ͼ��װ�õı�ţ�______
��3����ѡ��װ�õ�����˳��Ӧ�ǣ�����ӿڵ���ĸ�����ӽ���ʡ�ԣ���______��
���𰸡���������1�������ƺ������ƾ��ܺ�ˮ��Ӧ��
��2�����������ƺ���������ˮ��Ӧ�����𣺹������ƺ�ˮ��Ӧ��������������Һ��ͬʱ������������������������ˮ�������ⶨ���������������ȷ���������Ƶ�������ٷֺ�����
��3��װ�õ���װ˳����������ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ������������������������
����⣺��1�������ƺ������ƾ��ܺ�ˮ��Ӧ������ʽΪ��2Na2O2+2H2O=4NaOH+O2����Na2O+H2O=2NaOH���ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����Na2O+H2O=2NaOH��
��2��ʵ���ԭ���ǣ��������ƺ�ˮ��Ӧ��������������Һ��ͬʱ�����������������������л���ˮ������Ҫ��Ũ������ˮ��Ȼ���������ˮ�������ⶨ��������������ȷ���������Ƶ�������ٷֺ������ʿ�ѡ���װ��Ϊ�ݢ٢ܼ��ɣ��ʴ�Ϊ���ݢڢ٢ܣ�
��3������ˮ�������ⶨ��������ʱ������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ�����������������������������Ӵ���G���ӣ�A����B���ӣ�F�����ʴ�Ϊ����G���ӣ�D����C����A����B���ӣ�F����Ȼ��
������������Ҫ����ѧ�������Ƶ��������������������Լ�����ˮ�������ⶨ���������ķ������ѶȲ���
��2�����������ƺ���������ˮ��Ӧ�����𣺹������ƺ�ˮ��Ӧ��������������Һ��ͬʱ������������������������ˮ�������ⶨ���������������ȷ���������Ƶ�������ٷֺ�����
��3��װ�õ���װ˳����������ˮ��Ӧ������ˮ�������ⶨ���������������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ������������������������
����⣺��1�������ƺ������ƾ��ܺ�ˮ��Ӧ������ʽΪ��2Na2O2+2H2O=4NaOH+O2����Na2O+H2O=2NaOH���ʴ�Ϊ��2Na2O2+2H2O=4NaOH+O2����Na2O+H2O=2NaOH��
��2��ʵ���ԭ���ǣ��������ƺ�ˮ��Ӧ��������������Һ��ͬʱ�����������������������л���ˮ������Ҫ��Ũ������ˮ��Ȼ���������ˮ�������ⶨ��������������ȷ���������Ƶ�������ٷֺ������ʿ�ѡ���װ��Ϊ�ݢ٢ܼ��ɣ��ʴ�Ϊ���ݢڢ٢ܣ�
��3������ˮ�������ⶨ��������ʱ������ʢˮ���Լ�ƿ����һ��Ҫ�̽�����������ѹǿԭ����ˮ�ų�����Ͳ��ˮ�����������������������������Ӵ���G���ӣ�A����B���ӣ�F�����ʴ�Ϊ����G���ӣ�D����C����A����B���ӣ�F����Ȼ��
������������Ҫ����ѧ�������Ƶ��������������������Լ�����ˮ�������ⶨ���������ķ������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ