��Ŀ����
��������Ҫ�ɰ�������ɣ�ƽ��������������������Ϊ16%���ң�����ͨ�еļ�⵰���ʵķ����ǡ���ʽ��������--�Բ���ĺ���������һ��ϵ�������㵰���ʵĺ���������ǰ������ijЩ���ϸ��̷ۺ�α���̷��е����ʺ�������꣬�����˰��ո�������ͷ���ޡ��¼��ķ����������ع���������ꡰ��ʽ���������ļ��©����ͨ��������Ʒ�����Ӻ������ߴ�66.7%�������谷��C3H6N6������ɵ����ʺ������ļ����ɴ��ֵ�����ʯ��ׯ��¹�������̷ۡ��¼��ķ�����
��1���������ʵĺ�������16%�ƣ���ʽ�����������Ժ����������㵰���ʺ��������Ե�ϵ��ӦΪ ��
��2����ʽ����������Ҫԭ��Ϊ������Ʒ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬�����İ����������պ������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����ij����С��ͬѧ�ⶨҺ̬�̺�������ʵ��������£�
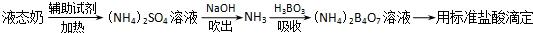
����岽�����£�
�����ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
����ӦҺת�Ƶ����Թ��У�
����ͼװ������Թ��м���10.00mL 40%��NaOH��Һ������ˮ���������ɵ�NH3����������H3BO3��Һ���մ�����NH3������װ��δ��������
����ȡ����ƿ���μ�ָʾ������0.1000mol/L�����Һ�ζ���
�����ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
�й����ݼ�¼���£�
��ش��������⣺
���ڲ�����ʵ��װ���У���Ҫ���ȵ������� �����������ƣ��������ܵ������� �����Թ�����������Ӧ�����ӷ���ʽ�� ����ȴˮӦ�� ������ĸ���ţ��ڽ��룮
�����4�ſհ���ʵ���Ŀ���� ��
�۵ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3����Ӧ�Ļ�ѧ����ʽΪ ��
�ܼ���10.00mLҺ̬���еĺ�����Ӧ�������ʽ����������� mL����Һ̬�̵ĺ�����Ϊ mg/mL��
��1���������ʵĺ�������16%�ƣ���ʽ�����������Ժ����������㵰���ʺ��������Ե�ϵ��ӦΪ
��2����ʽ����������Ҫԭ��Ϊ������Ʒ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬�����İ����������պ������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����ij����С��ͬѧ�ⶨҺ̬�̺�������ʵ��������£�
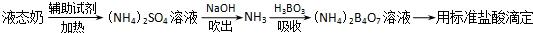
����岽�����£�
�����ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
����ӦҺת�Ƶ����Թ��У�
����ͼװ������Թ��м���10.00mL 40%��NaOH��Һ������ˮ���������ɵ�NH3����������H3BO3��Һ���մ�����NH3������װ��δ��������

����ȡ����ƿ���μ�ָʾ������0.1000mol/L�����Һ�ζ���
�����ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
�й����ݼ�¼���£�
| ��� | ��Ʒ�����Լ� | ����������� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45mL |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55mL |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50mL |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50mL |
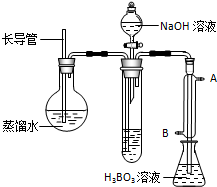
���ڲ�����ʵ��װ���У���Ҫ���ȵ�������
�����4�ſհ���ʵ���Ŀ����
�۵ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3����Ӧ�Ļ�ѧ����ʽΪ
�ܼ���10.00mLҺ̬���еĺ�����Ӧ�������ʽ�����������
��������1�����ݵ������к����������ϵ����
��2���ٸ��ݷ�Ӧԭ���ж���Ҫ���ȵ����������ݷ�Ӧװ��ѹ��������ܻᷢ��Σ�ռ���ȴʱ���ܷ���������������жϳ����ܵ����ã����Թ���������е������������������Һ��Ӧ���ɰ������ݴ�д����Ӧ�����ӷ���ʽ�����������ܲ�������ͨˮ�жϣ�
�ڸ���4�ſհ���ʵ���ܹ����������Լ���ʵ���������������������
�۸��ݷ�Ӧ��Ϊ��NH4��2B4O7���Ȼ��⣬����Ϊ������Ȼ��д����Ӧ�Ļ�ѧ����ʽ��
�ܸ���NԪ���غ㣬���ζ���Ӧ�ҳ�Nԭ����HCl�Ĺ�ϵʽ�����ݹ�ϵʽ���㣬ע����������ȡ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����
��2���ٸ��ݷ�Ӧԭ���ж���Ҫ���ȵ����������ݷ�Ӧװ��ѹ��������ܻᷢ��Σ�ռ���ȴʱ���ܷ���������������жϳ����ܵ����ã����Թ���������е������������������Һ��Ӧ���ɰ������ݴ�д����Ӧ�����ӷ���ʽ�����������ܲ�������ͨˮ�жϣ�
�ڸ���4�ſհ���ʵ���ܹ����������Լ���ʵ���������������������
�۸��ݷ�Ӧ��Ϊ��NH4��2B4O7���Ȼ��⣬����Ϊ������Ȼ��д����Ӧ�Ļ�ѧ����ʽ��
�ܸ���NԪ���غ㣬���ζ���Ӧ�ҳ�Nԭ����HCl�Ĺ�ϵʽ�����ݹ�ϵʽ���㣬ע����������ȡ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����
����⣺��1�������ʵĺ�����Ϊ16%�����Ժ����������㵰���ʺ��������Ե�ϵ��ӦΪ��
=6.25��
�ʴ�Ϊ��6.25��
��2���ٿ�ʽ����������Ҫԭ��Ϊ������Ʒ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬������Ҫ���ȵ���������ƿ������Բ����ƿ����ˮ�����������Թ��н����ɵİ������������Ȳ���ˮ������װ����ѹǿ���ӣ������ܷ�ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã����ݷ�Ӧԭ�������Թ��������������������Һ��Ӧ���ɰ�������Ӧ�����ӷ���ʽΪ��NH4++OH-
NH3��+H2O�������ܵ�ͨˮ����Ӧ��Ϊ����ͨˮ������B��ˮ��
�ʴ�Ϊ��Բ����ƿ����ȫ�ܣ���ֹװ����ѹ�����������Σ�գ���ֹ��ȴʱ������������NH4++OH-
NH3��+H2O��B��
�ڶ���ʹ��Ŀ�������������Լ���ʵ�����������������������հ���ʵ�飬���ĵ���������������ʵ����ƫ�ߣ�
�ʴ�Ϊ�����������Լ���ʵ������������������
�۵ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3��ͬʱ��Ӧ�������Ȼ�泥���Ӧ�Ļ�ѧ����ʽΪ����NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl��
�ʴ�Ϊ����NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl��
������������
-1.5ml=32.00ml=0.03200L����10ml��Һ̬�̵ĺ�����������Ϊmg����
2N����NH4��2B4O7��2HCl
28g 2mol
mg 0.03200L��0.1000mol/L
��m��2mol=28g��0.03200L��0.1000mol/L��
��ã�m=0.0448g=44.8mg��
��Һ̬�̵ĺ�����Ϊ
=4.480mg/mL��
�ʴ�Ϊ��32.00��4.480��
| 1 |
| 16% |
�ʴ�Ϊ��6.25��
��2���ٿ�ʽ����������Ҫԭ��Ϊ������Ʒ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬������Ҫ���ȵ���������ƿ������Բ����ƿ����ˮ�����������Թ��н����ɵİ������������Ȳ���ˮ������װ����ѹǿ���ӣ������ܷ�ֹװ����ѹ�����������Σ�գ���ȴʱ��ֹ������������ȫ�����ã����ݷ�Ӧԭ�������Թ��������������������Һ��Ӧ���ɰ�������Ӧ�����ӷ���ʽΪ��NH4++OH-
| ||
�ʴ�Ϊ��Բ����ƿ����ȫ�ܣ���ֹװ����ѹ�����������Σ�գ���ֹ��ȴʱ������������NH4++OH-
| ||
�ڶ���ʹ��Ŀ�������������Լ���ʵ�����������������������հ���ʵ�飬���ĵ���������������ʵ����ƫ�ߣ�
�ʴ�Ϊ�����������Լ���ʵ������������������
�۵ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3��ͬʱ��Ӧ�������Ȼ�泥���Ӧ�Ļ�ѧ����ʽΪ����NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl��
�ʴ�Ϊ����NH4��2B4O7+2HCl+5H2O=4H3BO3+2NH4Cl��
������������
| 33.45ml+33.55ml+33.50ml |
| 3 |
2N����NH4��2B4O7��2HCl
28g 2mol
mg 0.03200L��0.1000mol/L
��m��2mol=28g��0.03200L��0.1000mol/L��
��ã�m=0.0448g=44.8mg��
��Һ̬�̵ĺ�����Ϊ
| 44.8mg |
| 10mL |
�ʴ�Ϊ��32.00��4.480��
���������⿼���˵������к������ⶨ��������Ŀ�ѶȽϴ������漰��Ԫ�ػ����ʵ��ԭ������ѧ����ȣ����ؿ���ѧ����ʵ�鷽�����⡢Ԫ�ػ������֪ʶ���ѶȽϴ��״���Ϊ����Һ̬�̵ĺ�����ʱ�������ӦΪ3�εζ���ƽ��ֵ��ȥ1.5ml��������ʱ������������Ϊ1.5ml����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
��������Ҫ�ɰ�������ɣ�ƽ��������Ϊ16%���ң�ͨ�õĵ����ʲ��Է���--��ʽ������--��ͨ����������������㵰���ʺ�������Ҫԭ��Ϊ����ʳƷ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬�ֽ�İ����������պ������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����
��֪��1mol�����ܲ���1mol������
2NH3+4H3BO3����NH4��2B4O7+5H2O��
��NH4��2B4O7+2HCl+5H2O��2NH4Cl+4H3BO3
ij��ȤС�����������ʵ�飺
����I�� ��ȡ��Ʒ1.500g��
����II�����ԣ�
����õ�1L�����˰���������Ʒ��Һ��������ȡ10.00mL��250mL��ƿ�У���������ˮ��ָʾ������0.010mol/L������ζ����յ㣮
��1�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���������еζ���������Ʒ�е�����������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥ��������______ ���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯�� B����ƿ����Һ��ɫ�ı仯
��2���ζ�������±���ʾ��
| �ζ����� | ������Һ�������mL�� | ����Һ����� | |
| ��������mL�� | ĩ������mL�� | ||
| 1 | 10.00 | 0.00 | 14.90 |
| 2 | 10.00 | 0.10 | 15.05 |
| 3 | 10.00 | 0.50 | 17.00 |
��������Ҫ�ɰ�������ɣ�ƽ��������Ϊ16%���ң�ͨ�õĵ����ʲ��Է���--��ʽ������--��ͨ����������������㵰���ʺ�������Ҫԭ��Ϊ����ʳƷ������ʹ���һ����ȣ�ʹ�����ʷֽ⣬�ֽ�İ����������պ������������Һ�ζ�������������������Ի���ϵ������Ϊ�����ʺ�����
��֪��1mol�����ܲ���1mol������
2NH3+4H3BO3����NH4��2B4O7+5H2O��
��NH4��2B4O7+2HCl+5H2O��2NH4Cl+4H3BO3
ij��ȤС�����������ʵ�飺
����I ��ȡ��Ʒ1.500g��
����II ���ԣ�
����õ�1L�����˰���������Ʒ��Һ��������ȡ10.00mL��250mL��ƿ�У���������ˮ��ָʾ������0.010mol/L������ζ����յ㣮
��1�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���������еζ���������Ʒ�е�����������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥ��������______ ���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
��2���ζ�������±���ʾ��
����Ʒ�е�����������Ϊ______��
��֪��1mol�����ܲ���1mol������
2NH3+4H3BO3����NH4��2B4O7+5H2O��
��NH4��2B4O7+2HCl+5H2O��2NH4Cl+4H3BO3
ij��ȤС�����������ʵ�飺
����I ��ȡ��Ʒ1.500g��
����II ���ԣ�
����õ�1L�����˰���������Ʒ��Һ��������ȡ10.00mL��250mL��ƿ�У���������ˮ��ָʾ������0.010mol/L������ζ����յ㣮
��1�����ݲ������գ�
�ٵζ���������ˮϴ�Ӻ�ֱ�Ӽ���������еζ���������Ʒ�е�����������______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥ��������______ ���ƫ����ƫС������Ӱ�족����
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�______��
A���ζ�����Һ��ı仯 B����ƿ����Һ��ɫ�ı仯
��2���ζ�������±���ʾ��
| �ζ����� | ������Һ�������mL�� | ����Һ����� | |
| ��������mL�� | ĩ������mL�� | ||
| 1 | 10.00 | 0.00 | 14.90 |
| 2 | 10.00 | 0.10 | 15.05 |
| 3 | 10.00 | 0.50 | 17.00 |