��Ŀ����
1.��ͼ�Ǻϳ�ij�־�����άH������ͼ��

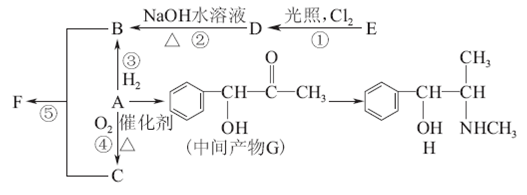
��֪��A��DΪ��������ͼ����G����Է�������Ϊ166�����к�̼57.8%������3.6%������Ϊ����G����NaHCO3��Һ��Ӧ�Һ��б������˴Ź������ױ���E��G�����о����������͵���ԭ�ӣ���E�������������͵���ԭ�ӵĸ�����Ϊ1��1��
��1���١��ݵķ�Ӧ��������Ϊ �� ��
��2��B������Ϊ�� ��F�Ľṹ��ʽΪ�� ��
��3��д�����л�ѧ����ʽ��
��
��
��
��4��F�ж���ͬ���칹��,д��ͬʱ������������������һ��ͬ���칹��Ľṹ��ʽ
a�����б����ұ�����ֻ��2��ȡ����
b������NaOH��Һ��Ӧ
c����Cu������O2��Ӧ�IJ����ܷ���������Ӧ
2.2-��-1��3-����ϩ�� �����Ʊ��ȶ���ԭ�ϡ�����˫���ϵ���ԭ�Ӻ��ѷ�
�����Ʊ��ȶ���ԭ�ϡ�����˫���ϵ���ԭ�Ӻ��ѷ�
��ȡ����Ӧ�����������ͨ��1��3-����ϩ��Cl2ֱ�ӷ�Ӧ�Ƶá�������2-��-1��3-����ϩ�ĺϳ�·�ߣ�

д��A��B�Ľṹ��ʽ���ü���ʽ��ʾ����A ��B

��֪��A��DΪ��������ͼ����G����Է�������Ϊ166�����к�̼57.8%������3.6%������Ϊ����G����NaHCO3��Һ��Ӧ�Һ��б������˴Ź������ױ���E��G�����о����������͵���ԭ�ӣ���E�������������͵���ԭ�ӵĸ�����Ϊ1��1��
��1���١��ݵķ�Ӧ��������Ϊ �� ��
��2��B������Ϊ�� ��F�Ľṹ��ʽΪ�� ��
��3��д�����л�ѧ����ʽ��
��
��
��
��4��F�ж���ͬ���칹��,д��ͬʱ������������������һ��ͬ���칹��Ľṹ��ʽ
a�����б����ұ�����ֻ��2��ȡ����
b������NaOH��Һ��Ӧ
c����Cu������O2��Ӧ�IJ����ܷ���������Ӧ
2.2-��-1��3-����ϩ��
 �����Ʊ��ȶ���ԭ�ϡ�����˫���ϵ���ԭ�Ӻ��ѷ�
�����Ʊ��ȶ���ԭ�ϡ�����˫���ϵ���ԭ�Ӻ��ѷ���ȡ����Ӧ�����������ͨ��1��3-����ϩ��Cl2ֱ�ӷ�Ӧ�Ƶá�������2-��-1��3-����ϩ�ĺϳ�·�ߣ�

д��A��B�Ľṹ��ʽ���ü���ʽ��ʾ����A ��B
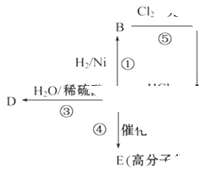
��18�֣���1���ӳɷ�Ӧ ������Ӧ ����1�֣���2��1,2-�������� ��2�֣�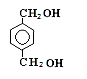 ��2�֣�
��2�֣�
��3�� ��2�֣�
��2�֣�
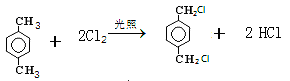 ��2�֣�
��2�֣�
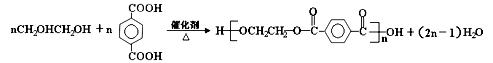 ��2�֣�
��2�֣�
��4��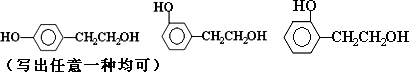 ��2�֣�
��2�֣� 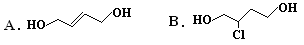 ����2�֣�
����2�֣�
 ��2�֣�
��2�֣���3��
 ��2�֣�
��2�֣� ��2�֣�
��2�֣�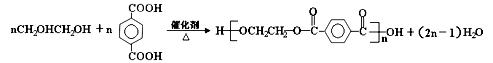 ��2�֣�
��2�֣���4��
 ��2�֣�
��2�֣� 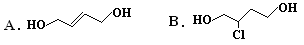 ����2�֣�
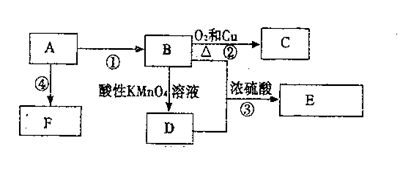
����2�֣�������������ݷ�Ӧ���������жϣ����Ǽӳɷ�Ӧ����B��±������±����������������Һ��ˮ������C�����Ը���C�Ļ�ѧʽ��֪��CӦ�����Ҷ�������B��1��2���������飬����A����ϩ��G����Է�������Ϊ166�����к�̼57.8%������3.6%������Ϊ������G��C��H��Oԭ�ӵĸ����ֱ���
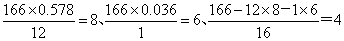 ����G�Ļ�ѧʽ��C8H6O4��G����NaHCO3��Һ��Ӧ�Һ��б�����˵�������Ȼ����˴Ź������ױ���G�����к����������͵���ԭ�ӣ�����G�ǶԱ������ᣬ�ṹ��ʽ��
����G�Ļ�ѧʽ��C8H6O4��G����NaHCO3��Һ��Ӧ�Һ��б�����˵�������Ȼ����˴Ź������ױ���G�����к����������͵���ԭ�ӣ�����G�ǶԱ������ᣬ�ṹ��ʽ��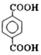 ��C�к���2���ǻ��������ܺ�G�������۷�Ӧ����H����H�Ľṹ��ʽ��
��C�к���2���ǻ��������ܺ�G�������۷�Ӧ����H����H�Ľṹ��ʽ��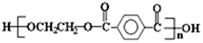 ��E�����к����������͵���ԭ�ӣ� E��������G����E�������������͵���ԭ�ӵĸ�����Ϊ1��1������E�Ľṹ��ʽӦ����
��E�����к����������͵���ԭ�ӣ� E��������G����E�������������͵���ԭ�ӵĸ�����Ϊ1��1������E�Ľṹ��ʽӦ����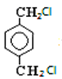 ����Eͨ��������Ӧ��������G��Dͨ����������E������A�Ľṹ��ʽ��
����Eͨ��������Ӧ��������G��Dͨ����������E������A�Ľṹ��ʽ��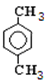 ��
�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ�������������ܽ�ȫ��ؿ���ѧ�����л���ѧ����֪ʶ����˼ά����������˼ά���������ѧ����Ӧ�������ʹ���Ч�ʡ�����Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ�������֮����ת����Ȼ��������������ü��ɡ�

��ϰ��ϵ�д�
�����Ŀ
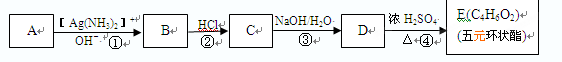 (1)X�ķ���ʽΪ ��
(1)X�ķ���ʽΪ ��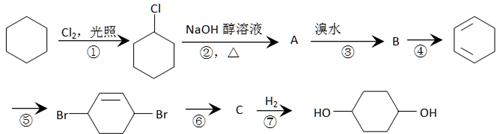
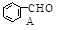 ��CH3CHO
��CH3CHO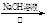
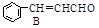 ��H2O
��H2O