��Ŀ����
ͭ������ϡ���ᷴӦ��Ҳ����Ũ���ᷴӦ����ͭ��һ��Ũ�����ᷴӦʱ���ɽ�����ʽ��ʾΪ��Cu+HNO3 �� Cu��NO3��2+NO��+NO2��+H2O ������ʽδ��ƽ��������2NO2 N2O4����
N2O4����
��1��0��004molCu��������ȫ�ܽ��Cuʧȥ�ĵ�������______________NA������õ���NO��NO2���ʵ�����ͬ����μӷ�Ӧ����������ʵ�����______________���ڱ�״���������ռ�NO��NO2�ļ���ƿ������ˮ�У�ͨ��һ������������ַ�Ӧ����������ʣ�࣬��ͨ��O2�����Ϊ____________��
��2������μӷ�Ӧ��Cu��HNO3�����ʵ���֮����3:10��д������ƽ�÷�Ӧ�����ӷ���ʽ__________ _��
 N2O4����
N2O4������1��0��004molCu��������ȫ�ܽ��Cuʧȥ�ĵ�������______________NA������õ���NO��NO2���ʵ�����ͬ����μӷ�Ӧ����������ʵ�����______________���ڱ�״���������ռ�NO��NO2�ļ���ƿ������ˮ�У�ͨ��һ������������ַ�Ӧ����������ʣ�࣬��ͨ��O2�����Ϊ____________��
��2������μӷ�Ӧ��Cu��HNO3�����ʵ���֮����3:10��д������ƽ�÷�Ӧ�����ӷ���ʽ__________ _��
��1��0��008NA����0��008mol���÷֣���2�֣���0��012mol��2�֣� ��44��8mL ��2�֣���
��2��3Cu + 10H++ 4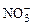 = 3Cu2++NO��+3NO2��+5H2O��3�֣�
= 3Cu2++NO��+3NO2��+5H2O��3�֣�
��2��3Cu + 10H++ 4
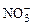 = 3Cu2++NO��+3NO2��+5H2O��3�֣�
= 3Cu2++NO��+3NO2��+5H2O��3�֣���

��ϰ��ϵ�д�
�����Ŀ
 + HNO2 �� HN3(������) + H2O (δ��ƽ)��
+ HNO2 �� HN3(������) + H2O (δ��ƽ)��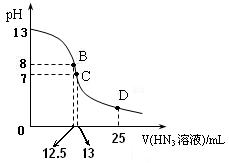
 D��ʱ��Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ
D��ʱ��Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ  (HN3
(HN3 ) ��
) ��  �������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��ѧ��
�������ݲ�����ʧ��ʣ�������Ҳ��ʹ�����ǵ�ľ����ȼ��ʯ����ҺҲ��Ϊ��ɫ��������Һ�屻ѹ��װ�â��С�װ�â��еĹ�����Ϊ��ɫ����ͬѧ�ݴ�д����AgNO3���ȷֽ���ܵ����ֻ�ѧ����ʽ��ѧ�� ����4AgNO3 2Ag2O��4NO2����O2����
����4AgNO3 2Ag2O��4NO2����O2����