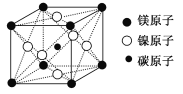
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
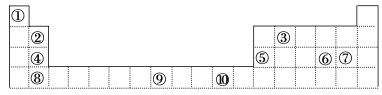
��ش��������⣺
��1����������d����Ԫ����___(����)���ܢݢޢߢ�����Ԫ���γɵ��ȶ������У����Ӱ뾶��С����_____(�����ӳƺ�)
��2������Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״���ӹ���Ϊ_____��
��3��ijԪ�ص����������Ų�ʽΪnsnnpn��1����Ԫ��ԭ�ӵ��������ӵŵ��Ӷ���Ϊ_____����ԭ�ӵĵ�һ������______�����Ų�ʽΪnsnnpn��2��ԭ��(������������������������С����)��
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��________��
��5������10��Ԫ���γɵ�����������Ӧˮ�����У�������ǿ����________(�ѧʽ)
��6�����и������Ŀռ乹����ͬ����_________
��NH3��H2O ��NH4+��H3O�� ��NH3��H3O�� ��O3��SO2 ��CO2��BeCl2 ��SiO44-��ClO4-��SO42-��BF3��Al2Cl6
���𰸡��� Al3+ ƽ���������� 1 ���� Be(OH)2+2NaOH=Na2BeO2+2H2O HClO4 �ۢܢݢ�
��������
��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪS����ΪCl����ΪCa����ΪFe����ΪCu��
(1)d��Ԫ�ذ����ڢ��塢��B�填��B��(��B������ϵԪ�ء��ϵԪ������f������)�����Ӳ�ṹ��ͬ�����Ӻ˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�����Ӱ뾶Խ��
(2)Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ����
(3)ijԪ�ص����������Ų�ʽΪnsnnpn+1������s�ܼ��������2�����ӣ���p�ܼ������ӣ���n=2������ȫ�������������ȫ�յ��ӹ��͵�ԭ�ӱȽ��ȶ�����һ�����ܸ���ͬ��������Ԫ�صģ�
(4)Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���Ԫ�آڵ���������Be(OH)2��NaOH��Ӧ����Na2BeO2��ˮ��
(5)����10��Ԫ���γɵ�����������Ӧˮ�����У�������ǿ���Ǹ����
(6)��������ԭ�ӵŵ��Ӷ�����۲���Ӷ����жϡ�
��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪBe����ΪC����ΪMg����ΪAl����ΪS����ΪCl����ΪCa����ΪFe����ΪCu��
(1)���Т�����d��Ԫ�أ����ڵ��Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӻ�����Ӳ�Խ�࣬���Ӱ뾶Խ�������Ӱ뾶�ɴ�С��˳��Ϊ��S2->Cl->Ca2+>Mg2+>Al3+���ɼ����Ӱ뾶��С��ΪAl3+��
(2)Ԫ�آٵ�6��ԭ����Ԫ�آ۵�6��ԭ���γɵ�ij�ֻ�״����Ϊ���������ӹ���Ϊƽ���������Σ�
(3)ijԪ�ص����������Ų�ʽΪnsnnpn+1������s�ܼ��������2�����ӣ���p�ܼ������ӣ���n=2�����������Ų�ʽΪ2s22p3��2s�����2������Ϊ�¶Ե��ӣ���Ԫ�ص�2p���Ϊ��������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ���NԪ�صĵ�һ�����ܴ��ڵ����Ų�ʽΪnsnnpn+2��Oԭ�ӵĵ�һ�����ܣ�
(4)Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���Ԫ�آڵ���������Be(OH)2��NaOH��Ӧ����Na2BeO2��ˮ����Ӧ����ʽΪ��Be(OH)2��2NaOH=Na2BeO2��2H2O��
(5)����10��Ԫ���γɵ�����������Ӧˮ�����У�������ǿ����HClO4��
(6)��NH3��Nԭ�ӹµ��Ӷ���=![]() =1���۲���Ӷ���=1+3=4����˿ռ乹��Ϊ�����Σ�H2O��Oԭ�ӹµ��Ӷ���=
=1���۲���Ӷ���=1+3=4����˿ռ乹��Ϊ�����Σ�H2O��Oԭ�ӹµ��Ӷ���=![]() =2���۲���Ӷ���=2+2=4���ռ乹��ΪV�Σ����߿ռ乹�Ͳ���ͬ���ٲ����ϣ�
=2���۲���Ӷ���=2+2=4���ռ乹��ΪV�Σ����߿ռ乹�Ͳ���ͬ���ٲ����ϣ�
��NH4+��Nԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ�H3O+��Oԭ�ӹµ��Ӷ���=
=0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ�H3O+��Oԭ�ӹµ��Ӷ���=![]() =1���۲���Ӷ���=1+3=4���ռ乹��Ϊ�����Σ����߿ռ乹�Ͳ���ͬ���ڲ����ϣ�
=1���۲���Ӷ���=1+3=4���ռ乹��Ϊ�����Σ����߿ռ乹�Ͳ���ͬ���ڲ����ϣ�
��NH3Ϊ�����Σ�H3O+Ҳ�������Σ����߿ռ乹����ͬ���۷��ϣ�
��O3��Oԭ�ӹµ��Ӷ�=![]() =1���۲���Ӷ���=1+2=3���ռ乹��ΪV�Σ�SO2��Sԭ�ӹµ��Ӷ�=
=1���۲���Ӷ���=1+2=3���ռ乹��ΪV�Σ�SO2��Sԭ�ӹµ��Ӷ�=![]() =1���۲���Ӷ���=1+2=3���ռ乹��ΪV�Σ����߿ռ乹����ͬ���ܷ��ϣ�
=1���۲���Ӷ���=1+2=3���ռ乹��ΪV�Σ����߿ռ乹����ͬ���ܷ��ϣ�
��CO2Ϊֱ���νṹ��BeCl2��Beԭ��û�й¶Ե��ӡ��۲���Ӷ���=0+2=2���ռ乹��Ϊֱ���Σ����߿ռ乹����ͬ���ݷ��ϣ�
��SiO44-��Siԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ�ClO4-��Clԭ�ӹµ��Ӷ���=
=0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ�ClO4-��Clԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=0+4=4���ռ乹��Ϊ�������Σ�SO42-��Sԭ�ӹµ��Ӷ���=
=0���۲���Ӷ���=0+4=4���ռ乹��Ϊ�������Σ�SO42-��Sԭ�ӹµ��Ӷ���=![]() =0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ����߿ռ乹����ͬ�����ϣ�
=0���۲���Ӷ���=0+4=4���ռ乹��Ϊ���������Σ����߿ռ乹����ͬ�����ϣ�
��BF3������Bԭ����û�йµ��Ӷԡ��۲���Ӷ���=0+3=3���ռ乹��Ϊƽ���������Σ�Al2Cl6��Alԭ���γ�4�����ۼ�������ƽ���νṹ�����߿ռ乹�Ͳ���ͬ���߲����ϣ�
�ʷ���Ҫ�������Ǣۢܢݢޡ�

 �ο�������ϵ�д�
�ο�������ϵ�д� ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д�����Ŀ����ѧʵ�������ƻ�������������ܿ�����Ԥ�ڵ��������й���ʵ������ķ�������ȷ���ǣ� ��
ѡ�� | A | B | C | D |
װ�� |
|
|
|
|
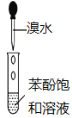
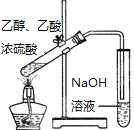
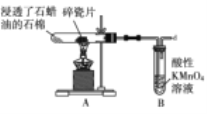
���� | ��Ͳ��δ������״Һ�� | �Թ����к�ɫ��������δ�������� | �Թ���δ������ɫ���� | NaOH��ҺҺ���� δ������״Һ�� |
ԭ�� | ����������δ��Ӧ | ��Ӧδ���ɵ����� | ����Ũ�� �ϴ� | ����ˮ�� |
A. AB. BC. CD. D