��Ŀ����
��A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ����������������֪��A��C��B��D�ֱ�λ��ͬ���壬��B��D������֮����A��C������֮�͵�2����E��ͬ����Ԫ����ԭ�Ӱ뾶��С��
��1��A2B��A2D�ķе�ϸ����� ���ѧʽ������ԭ���� ��
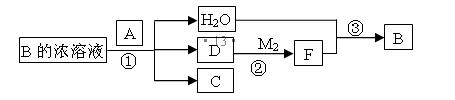
��2���ö��ʯī�缫�����з�̪��C��E�γɵĻ�����ı�����Һ����ͨ��Դһ��ʱ��� ������缫���ƣ��������ֺ�ɫ����һ���ĵ缫��ӦʽΪ ������ ����ü����ò�������ܷ�Ӧ����ʽΪ ��
��1��A2B��A2D�ķе�ϸ����� ���ѧʽ������ԭ���� ��
��2���ö��ʯī�缫�����з�̪��C��E�γɵĻ�����ı�����Һ����ͨ��Դһ��ʱ��� ������缫���ƣ��������ֺ�ɫ����һ���ĵ缫��ӦʽΪ ������ ����ü����ò�������ܷ�Ӧ����ʽΪ ��
��1��H2O �����Ӽ��γ��������
��2������ ��ʪ��ĵ��۵⻯����ֽ��
��ʪ��ĵ��۵⻯����ֽ��
2NaCl + 2H2O 2NaOH + H2��+Cl2��
2NaOH + H2��+Cl2��
��2������
 ��ʪ��ĵ��۵⻯����ֽ��
��ʪ��ĵ��۵⻯����ֽ��2NaCl + 2H2O
 2NaOH + H2��+Cl2��
2NaOH + H2��+Cl2����

��ϰ��ϵ�д�
�����Ŀ
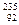 Uֻռ0.724������Ũ����ָ�����Ԫ����
Uֻռ0.724������Ũ����ָ�����Ԫ����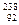 U��ѧ���ʵIJ�ͬ�����߷���
U��ѧ���ʵIJ�ͬ�����߷��� A�У�X��YԪ�ص�������Ϊ7��16����A����Ϊ ɫ�� ���ѧʽ����
A�У�X��YԪ�ص�������Ϊ7��16����A����Ϊ ɫ�� ���ѧʽ����