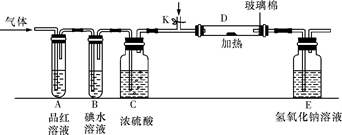
��Ŀ����
A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶C> D >A>B����֪��A��Bλ��ͬһ���ڣ�A��Dλ��ͬһ���壻Dԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ�Dԭ��������Ӳ��ϵĵ�������Cԭ��������Ӳ��ϵĵ�������4�����Իش�
��1��C��DԪ�طֱ��ǣ� C �� , D ��
��2��������Ԫ���У��ڳ��³�ѹ�µ�Һ�����̬�⻯����ȶ����ɴ�С��˳��Ϊ ��
��3��A��B�γɵ���ԭ�ӷ��ӵĵ���ʽ�� �� �� B��C�γɵ�ԭ�Ӹ�����Ϊ1 ��1�Ļ�����ĵ���ʽΪ �� ��Ԫ��D������������ �� ���塣
(4) д����D��������Ϊԭ�Ϲ�ҵ��ȡ����D�Ļ�ѧ����ʽΪ ��
��1��C��DԪ�طֱ��ǣ� C �� , D ��
��2��������Ԫ���У��ڳ��³�ѹ�µ�Һ�����̬�⻯����ȶ����ɴ�С��˳��Ϊ ��
��3��A��B�γɵ���ԭ�ӷ��ӵĵ���ʽ�� �� �� B��C�γɵ�ԭ�Ӹ�����Ϊ1 ��1�Ļ�����ĵ���ʽΪ �� ��Ԫ��D������������ �� ���塣
(4) д����D��������Ϊԭ�Ϲ�ҵ��ȡ����D�Ļ�ѧ����ʽΪ ��
�ԣ�1�� Na ��1�֣� , Si ��1�֣� ��
��2�� H2O>CH4>SiH4 ��2���� ��
��3��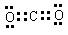 ��
�� 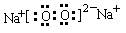 �� ԭ�� ������1����
�� ԭ�� ������1����
(4) SiO2 + 2C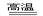 Si + CO�� ��2����
Si + CO�� ��2����
��2�� H2O>CH4>SiH4 ��2���� ��
��3��
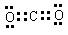 ��
�� 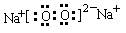 �� ԭ�� ������1����
�� ԭ�� ������1����(4) SiO2 + 2C
 Si + CO�� ��2����
Si + CO�� ��2���� 
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ