��Ŀ����
�����Ը��������Һ�м���Na2O2��ĩ�����������Һ��ɫ�����з�����Ӧ�����ӷ���
ʽΪ��2MnO4-+16H+ + 5Na2O2 ==== 2Mn2+ +5O2��+ 8H2O + 10Na+ �����ж���ȷ����:
A�������������������Na2O2���������������ǻ�ԭ��
B����Ӧ����������ֻ������
C������Ӧ������״����2.24 L O2 ʱ����Ӧת�Ƶĵ���Ϊ0.1mol
D������������Һ�μ�Ũ������Եõ����Ը��������Һ
B
����:��

��ϰ��ϵ�д�
 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д�
�����Ŀ
A��B��C��D��E��Ϊ�л������A�Ƿ���ʽΪC5H10O��ֱ�������B��NaHCO3��Һ��ȫ��Ӧ�������ʵ���֮��Ϊ1��2������֮��Ĺ�ϵ��ͼ��ʾ����ʾ��RCH=CHR�������Ը��������Һ�з�Ӧ����RCOOH��R��COOH������R��R��Ϊ���������������������ǣ�������
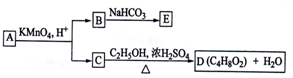

| A��B�Ľṹ��ʽΪHOOC-CH2-COOH | B��C���Ҵ�������������Ӧ | C��A����������������Һ�����кͷ�Ӧ | D��E�ķ���ʽΪC3H2O4Na2 |








