��Ŀ����
��ij��ͭ��ʯ[��Ҫ�ɷ���FeCuSi3O13(OH)4��������SiO2��CaCO3]Ϊԭ���Ʊ�CuSO4��5H2O���������£�
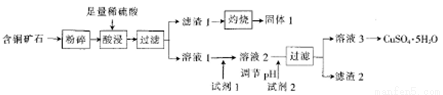
��֪����Լ��ɷֺͼ۸����±���ʾ��
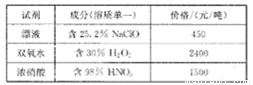
��ش��������⣺
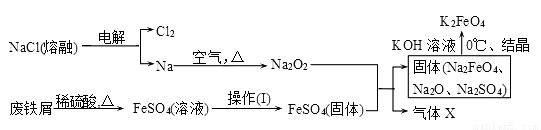
��1����ͭ��ʯ�����Ŀ����_______��
��2����������Һ�г���Cu2+�⣬�����еĽ�����������_______��
��3������1����NaOH��Һ�����ӷ���ʽΪ__________��
��4�����������Ϣ��֪����ѡ�õ��Լ�1������Ϊ_______��������Լ�ʱ��������Ӧ�����ӷ���ʽΪ_________��
��5���Լ�2 ����ѡ����������е�______������2��һ�����е�����Ϊ______(�ѧʽ����
A. Cu B.CuO C.Cu(OH)2 D.Fe
��6��CuSO4��5H2O���ڵ�⾫��ͭʱ��������ͨ��9.632��103C�ĵ�������������ܽ��ͭΪ16.0g�����������Һ��ԭ��ҺΪ1 L����ǡ����CuSO4����������������������_____g��ԭ���Һ��CuSO4��Ũ��Ϊ__ ����֪һ�����ӵĵ���Ϊ1.6��10-19C��
��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ