��Ŀ����
����Ŀ�����������е�������ԭ�����α�����ԭ�ӻ����ȡ��ʱ�����γɵĻ�������������������Ҫ��N2H4(��)��HN3(�������⣬����ˮ��Ϊ������)��NH2OH(�ǰ�)��ˮ����(N2H4��H2O)���Ʊ���������(NaN3)��ԭ�ϣ���������������������ȫ��������������巢������ԭ�ϡ������ǹ�ҵˮ���·��Ʊ��������ƵĹ������̡�
���ϣ���ˮ�����ж��Ҳ��ȶ�������ǿ��ԭ�Ժ�ǿ���ԣ�
���й����ʵ������������±���
���� | �״� | ˮ���� | ��������� | �������� |
�۵�(��) | -97 | -40 | -17 | 275(410�棺�ֽ�) |
�е�(��) | 64.7 | 118.5 | -12 | �� |
�ش��������⣺
I.�ϳ�ˮ���¡�ʵ���Һϳ�ˮ����װ������ͼ��ʾ��NaClO������Һ������CO(NH2)2ˮ��Һ��400C���·�Ӧһ��ʱ�����Ѹ��������1100�������Ӧ�����Ƶ�ˮ���¡�
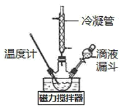
(1)ʵ����ͨ����Һ©��������ƿ�л����μ�NaClO������Һ�����ܷ���μӵ�ԭ����______________����ȡN2H4H2O�����ӷ���ʽΪ_______________________��
II.�Ʊ��������ơ�ʵ���ҿ�������ͼ����ʾ��װ�ü�ҩƷ�Ʊ��������ơ�
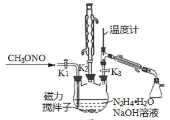
(2)�ٸ���ʵ�鷢���¶���20�����ҷ�Ӧ��ת������ߣ���˿��ȡ�Ĵ�ʩ��_______________������������A��Һʱ��װ��������K1��K2��K3�Ŀ��������_______________________��
��д���÷����Ʊ��������ƵĻ�ѧ����ʽ��________________________��
(3)��������B��Һ��õ������Ʋ�Ʒ��ʵ�鲽��Ϊ____________________����ѹ���ˣ��������Ҵ�ϴ��23�κ��
(4)���������У�����ĵ������Ƴ�ʹ�ô���������Һ�����������������£����߷�Ӧ�������������塣������6.5gNaN3�������������0.5molL��NaClO��Һ_____________mL��
III.�ǰ�(NH2OH)��һ�ֻ�ԭ��������ͨ�����й��̵õ�����ϩ��N2O4�����Ϻ��գ������ӳɷ�Ӧ���õ�������A��A�ṹ�Գƣ�������ͬԪ�ص�ԭ�ӻ�ѧ������ͬ��A��ijŨ�ȵ�������Һ�л��ɵõ�������B��ͬʱ�õ�CO��CO2��ɵĻ������(��������ܶ�Ϊ18)����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų���B��Һ���м��õ�NH2OH��
(5)NH2OH���������ԣ������ᷴӦ�����Σ����������ӵĵ���ʽΪ_______________��
(6)д��A��B�Ļ�ѧ����ʽ________________________��
���𰸡���ֹ������NaClO��Һ����ˮ���� ClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32 20����ˮԡ����ͨ������� �ر�K1��K2����K3 CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O �����ᾧ 100 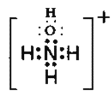 CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4
CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4
��������
ʵ���Һϳ�ˮ����װ������ͼ��ʾ��NaClO������Һ������CO(NH2)2ˮ��Һ��400�����·�Ӧһ��ʱ�����Ѹ��������1100�������Ӧ�����Ƶ�ˮ���£�������Ӧ�����ӷ���ʽΪ��ClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32��NaNO2��Һ��CH3OH�����������·�����Ӧ2CH3OH+2NaNO2+H2SO4(Ũ)=2CH3ONO+Na2SO4+2H2O�������������(CH3ONO)�����������(CH3ONO)����������ˮ���»��Һ��Ӧ���ɼ״��͵������ƣ���Ӧ����ʽΪ��CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O����ϼ״��͵������Ƶ��۷е㲻ͬ����������ķ���������״��͵������ƣ���B��ҺΪ����������Һ�������нᾧ�����ˡ�ϴ�ӡ������ò�Ʒ�������ƣ��ݴ˷������I��II��
III����ϩ��N2O4�����Ϻ��գ������ӳɷ�Ӧ����A��A�Ľṹ�Գƣ�������ͬԪ�ص�ԭ�ӻ�ѧ������ͬ��������ӦΪ��CH2=CH2+ N2O4��CH2NO2CH2NO2���õ�������AΪCH2NO2-CH2NO2��A��ijŨ�ȵ�������Һ�л��ɵõ�������B��ͬʱ�õ�CO��CO2��ɵĻ������(��������ܶ�Ϊ18)�����Ϻ������ƽ����Է�������Ϊ18��2=36������ʮ����˷���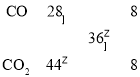 ����
����![]() ����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%����B�к���Sԭ�Ӹ���Ϊx��Oԭ�Ӹ���Ϊy�� B��Ħ������ΪM����
����������BΪ����̼Ԫ�ص������Σ��������Ԫ�ص����������ֱ�Ϊ19.51%��58.54%����B�к���Sԭ�Ӹ���Ϊx��Oԭ�Ӹ���Ϊy�� B��Ħ������ΪM����![]() ��100%=19.51%��
��100%=19.51%��![]() ��100%=58.54%����ʽ����ɵã�
��100%=58.54%����ʽ����ɵã�![]() =
=![]() ����B�к���1��Sԭ��ʱ��Oԭ��Ϊ6�����������������ԭ�Ӹ�����Ϊ1��4��˵�������ӽṹ�к������� B����Է�������M=
����B�к���1��Sԭ��ʱ��Oԭ��Ϊ6�����������������ԭ�Ӹ�����Ϊ1��4��˵�������ӽṹ�к������� B����Է�������M=![]() =164����ΪNH2OH���������ԣ��ɽ��H+�õ�NH3OH+����BӦΪ(NH3OH)2SO4��(NH3OH)2SO4��Һ���з�Ӧ�õ�NH2OH��NH4+������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų�����Ӧ����ʽΪCH3CH2NO2+ H2SO4��(NH3OH)2SO4+C4H6O2���ݴ˷������
=164����ΪNH2OH���������ԣ��ɽ��H+�õ�NH3OH+����BӦΪ(NH3OH)2SO4��(NH3OH)2SO4��Һ���з�Ӧ�õ�NH2OH��NH4+������A����CH3CH2NO2�������Ƶķ�Ӧ��Ҳ�ܵõ�B����û������ų�����Ӧ����ʽΪCH3CH2NO2+ H2SO4��(NH3OH)2SO4+C4H6O2���ݴ˷������
I��(1)����������Ϣ��ˮ�����ж��Ҳ��ȶ�������ǿ��ԭ�Ժ�ǿ���ԣ�NaClO����ǿ�����ԣ�������ˮ���£�Ϊ��ֹ������NaClO����ˮ���£����ܷ���μӣ�NaClO��CO(NH2)2�ڼ��������·�Ӧ����ˮ���¡������Ӻ�̼������ӣ����ӷ���ʽClO+CO(NH2)2+2OH=Cl+N2H4H2O+CO32��
II��(2)�ٸ���ʵ�鷢���¶���20�����ҷ�Ӧ��ת������ߣ����Ǹ÷�Ӧ���ڷ��ȷ�Ӧ����˿��Բ�ȡ�Ĵ�ʩ�ǣ�20����ˮԡ����ͨ������ȣ�����������A��Һʱ����ҺA��������ĺ�������˳���ǣ��ر�K1��K2����K3��
�ڸ��ݷ�����֪���Ʊ��������ƵĻ�ѧ����ʽ��CH3ONO+N2H4H2O+NaOH�TCH3OH+NaN3+3H2O��
(3)��������B��Һ��õ������Ʋ�Ʒ��ʵ�鲽��Ϊ�����ᾧ����ѹ���ˣ��������Ҵ�ϴ��23�κ��
(4)����������NaN3Ӧ�ñ�����Ϊ����N2��ͬʱClO����ԭΪCl�����ÿ��NaN3�ڷ�Ӧ����Ҫʧȥ1�����ӣ�ÿ��ClO�ڷ�Ӧ�п��Եõ�2�����ӣ����ߵ����ʵ���֮��Ϊ2:1 �������ķ�ӦΪ��ClO+2N3-+2H+= Cl+3N2��+H2O��NaN3�����ʵ���n=![]() =0.1mol������ҪClO�����ʵ���Ϊ0.05mol�������Ҫ����������Һ���V=
=0.1mol������ҪClO�����ʵ���Ϊ0.05mol�������Ҫ����������Һ���V=![]() =0.1L=100mL��
=0.1L=100mL��
III��(5)�ǰ�(NH2OH)�ǰ�����������Կ��������е�һ����ԭ�ӱ��ǻ�ȡ�����γɵ����ʣ�NH2OH���������ԣ���Ȱ�����HCl����NH4Cl�ķ�Ӧ�����ǰ�(NH2OH)�����ᷴӦ�������ӻ�����HONH3Cl�������ӻ�����NH3OHCl��[NH3HO]+��Cl-���ɣ����������ӵĵ���ʽΪ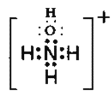 ��
��
(6)�����⣬A��ijŨ�ȵ�������Һ�л��ɵõ�������B�����ɵ�CO��CO2�����ʵ���֮��![]() ����Ӧ����ʽΪ��CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4��
����Ӧ����ʽΪ��CH2NO2CH2NO2+H2SO4��CO+CO2+(NH3OH)2SO4��