��Ŀ����
����úΪ��Ҫԭ�ϵĺϳɰ���ҵ�У�ԭ����������������������ã�
��C��H2O(g)![]() CO��H2��
CO��H2��
��CO��H2O(g)![]() CO2��H2��
CO2��H2��
(1)��֪CO(g)��1/2O2(g)��CO2(g)����H����283.0 kJ/mol��
H2(g)��1/2O2(g)��H2O(g)����H����285.8 kJ/mol��д������CO��H2O(g)��Ӧ���Ȼ�ѧ����ʽ��
________________
(2)�ӷ�Ӧ������з����H2�ķ���ͨ�����Լ�Һϴ�������ݸù�ҵ������ʵ�ʷ��������ѡ������������Һ��Ϊ���ռ���________��������________��
A������������Һ
B����ˮ
C��ʯ��ˮ��ʯ����
(3)��ʵ����ģ��������Ӧ�ڣ�830��ʱ��1 L��������װ��CO��H2O(g)��2 molʹ֮��Ӧ���ﵽƽ��ʱ���������CO2��Ũ��Ϊ1 mol/L������830��ʱ�÷�Ӧ��ƽ�ⳣ����
�𰸣�
������
������
����(1)CO(g)��H2O(g)��CO2(g)��H2(g)����H��2.8 kJ/mol
����(2)B���ó��зḻ�İ�ˮ��Դ�����պ��γ�̼泥���һ�ֻ��ʣ���ȡ�ýϺõ��ۺ�Ч�森
����(3)1

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ

 2NH3(g) ��H <0
2NH3(g) ��H <0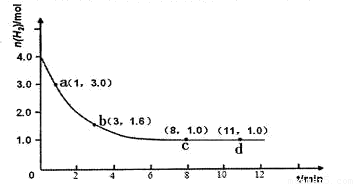
 CO+H2����CO+H2O(g)
CO+H2����CO+H2O(g)  2NH3(g)
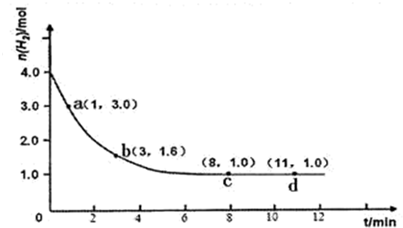
��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
2NH3(g)
��H<0��ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��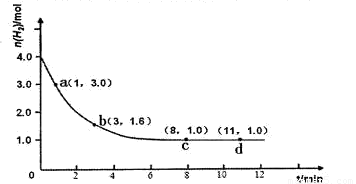
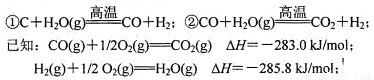

 CO+H2����CO+H2O��g��
CO+H2����CO+H2O��g�� CO2+H2��
CO2+H2�� O2��g���TCO2��g����H=-283.0KJ/mol��
O2��g���TCO2��g����H=-283.0KJ/mol�� O2��g��=H2O��g����H=-285.8KJ/mol��
O2��g��=H2O��g����H=-285.8KJ/mol��
 CO+H2����CO+H2O��g��
CO+H2����CO+H2O��g�� CO2+H2��
CO2+H2�� O2��g���TCO2��g����H=-283.0KJ/mol��
O2��g���TCO2��g����H=-283.0KJ/mol�� O2��g��=H2O��g����H=-285.8KJ/mol��
O2��g��=H2O��g����H=-285.8KJ/mol��