��Ŀ����
��ҵ�ϳɰ��ķ�ӦΪ��N2(g)+3H2(g) 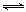 2NH3(g) ��H <0
2NH3(g) ��H <0
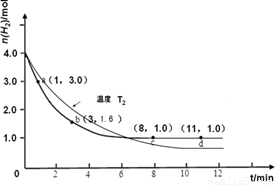
ijʵ�齫3.0 mol N2(g)��4. 0 mol H2(g)�����ݻ�Ϊ10L���ܱ������У����¶�T1�·�Ӧ�����H2�����ʵ����淴Ӧʱ��ı仯����ͼ��ʾ��
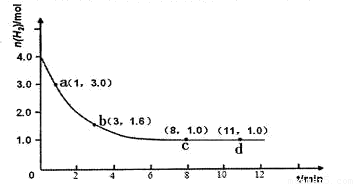
��1����Ӧ��ʼ3min�ڣ�H2��ƽ����Ӧ����Ϊ ��
��2������������ºϳɰ���Ӧ�Ļ�ѧƽ�ⳣ����д��������̣��������2λ��Ч���֣���
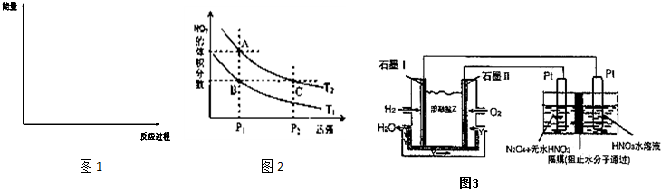
��3�����ı��¶�ΪT2 ( T2С��TI���ٽ���ʵ�飬���ڴ����ͼ�л���H2�����ʵ����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
��4������úΪ��Ҫԭ�ϵĺϳɰ���ҵ�У�ԭ����������������������ã�
��C+H2O(g)
 CO+H2����CO+H2O(g)
CO+H2����CO+H2O(g)  CO2+H2��
CO2+H2��
��֪��CO(g)+1/2O2(g)=CO2(g) ��H=��283.0kJ/mol
H2(g)+1/2O2(g)=H2O(g) ��H=��241.8kJ/mol
д������CO��H2O(g)��Ӧ���Ȼ�ѧ����ʽ�� ��
��5���ϳɰ���ҵ�У�ԭ����(N2��H2��������CO��NH3���ڽ���ϳ���֮ǰ���ô��������ͭ��I����Һ������CO���䷴ӦΪ��
CH3COO[Cu(NH3)2]+CO+NH3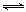 CH3COO[Cu(NH3)3]•CO
��H<0
CH3COO[Cu(NH3)3]•CO
��H<0
д�����CO�����ʵ�����һ���ʩ�� ��
��16�֣�
��1��0.080mol/(L��min) ��3�֣���λ����Ч���ִ�©�Ͽ�1�֣�
��2������5�֣��⣺�����⣬ƽ��ʱH2��Ũ��Ϊ0.10 mol/L
N2(g) + 3H2(g)
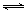 2NH3(g)
2NH3(g)
��ʼŨ�ȣ�mol/L�� 0.30 0.40 0
ת��Ũ�ȣ�mol/L�� 0.10 0.30 0.20
ƽ��Ũ�ȣ�mol/L�� 0.20 0.10 0.20 ��1�֣�
K=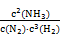 =
=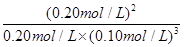 =2.0��102
(L/mol)2
=2.0��102
(L/mol)2
�𣺸������ºϳɰ���Ӧ�Ļ�ѧƽ�ⳣ��Ϊ2.0��102 (L/mol)2��
��4�֣����д��빫ʽ��������ֵ��1�֣����2�֣�������ֵ������λ��λ����Ч���ִ���Ͽ�1�֣� Kֵ������λ���۷֣�
��3����2�֣�ע��������㡢б�ʡ�ƽ��㣨t>8min,n(H2)<1.0mol��Ҫ���д���ÿ��Ҫ�ؿ�1��ֱ��0�֣�����ע�¶Ȳ��۷֣�
��4��CO(g)+H2O(g)=CO2(g)+H2(g) ��H=��41.2 kJ/mol
��4�֣�״̬��©��С��0�֣����У�����ʽ2�֣���H 2�֣���©��λ��1�֡����������������������֣�
��5����ѹ�����£�����Ũ��ˮ ��������������ͭ(��)��ҺŨ�ȣ���ʱ����CH3COO[Cu(NH3)3]��CO����дһ�㣬�ô��������֣�2�֣����������֣�
��������
�����������1����ͼ����ʼʱ����Ϊ4.0mol��3minʱ����Ϊ1.6mol�����������ݻ�Ϊ10L���������ı仯Ũ��Ϊ(
4.0mol��1.6mol)/10L=0.24mol/L����v(H2)=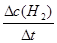 =
= = 0.080mol/(L•min)����2���⣺�����⣬��ʼ��ƽ��ʱH2��Ũ�ȷֱ�Ϊ0.30mol/L��0.10 mol/L��
= 0.080mol/(L•min)����2���⣺�����⣬��ʼ��ƽ��ʱH2��Ũ�ȷֱ�Ϊ0.30mol/L��0.10 mol/L��
N2(g) + 3H2(g) 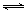 2NH3(g)
2NH3(g)
��ʼŨ�ȣ�mol/L�� 0.30 0.40 0
ת��Ũ�ȣ�mol/L�� 0.10 0.30 0.20
ƽ��Ũ�ȣ�mol/L�� 0.20 0.10 0.20
K��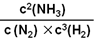 =
=
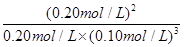 =
2.0��102 (L/mol)2
=
2.0��102 (L/mol)2
�𣺸������ºϳɰ���Ӧ�Ļ�ѧƽ�ⳣ��Ϊ2.0��102 (L/mol)2��
��3����ͼҪ�㣺��T1��T2�������¶ȣ���Ӧ���ʼ�С����λʱ�����������������ʵ�����С���ﵽƽ��ʱ��������������Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ��������Ӧ�����ƶ�����ƽ��ʱ���������ʵ�����С����4�����ݸ�˹���ɣ���һ���Ȼ�ѧ����ʽ��ȥ�ڶ����Ȼ�ѧ����ʽ����CO(g)+H2O(g)=CO2(g)+H2(g) ��H=��41.2
kJ/mol����5������CH3COO[Cu(NH3)2]+CO+NH3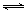 CH3COO[Cu(NH3)3]•CO������Ӧ�����������С�ķ��ȷ�Ӧ�����������������ͭ��I����ҺŨ�Ȼ�����Ũ��ˮ����ʱ����CH3COO[Cu(NH3)3]•CO������ѹǿ�������¶ȵȴ�ʩ������ʹƽ��������Ӧ�����ƶ������CO�������ʻ�ת���ʡ�
CH3COO[Cu(NH3)3]•CO������Ӧ�����������С�ķ��ȷ�Ӧ�����������������ͭ��I����ҺŨ�Ȼ�����Ũ��ˮ����ʱ����CH3COO[Cu(NH3)3]•CO������ѹǿ�������¶ȵȴ�ʩ������ʹƽ��������Ӧ�����ƶ������CO�������ʻ�ת���ʡ�
���㣺���黯ѧ��Ӧԭ�����漰ƽ����Ӧ���ʵļ��㡢��ѧƽ�ⳣ���ļ�����̡������¶ȶԻ�ѧ��Ӧ���ʺ�ƽ���ƶ���Ӱ��ͼ��˹���ɡ��Ȼ�ѧ����ʽ����д����߷�Ӧ��ת���ʵĴ�ʩ�ȡ�

| T/K | 303 | 313 | 323 | 353 |
| NH3������/��10-6 mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
| 3 |
| 2 |
�ش��������⣺
��1���÷�Ӧ�ڽϵ��¶����ܷ��Է����У�
��2����323K��353K�����������������ٵ�ԭ��
��3���뻭��������Ӧ���д�����������������·�Ӧ��������ϵ�����仯ʾ��ͼ1�������б�Ҫ��ע��
��4����ҵ�ϳɰ��ķ�ӦΪN2��g��+3H2��g��?2NH3��g���������ݻ�Ϊ2.0L���ܱ������г���0.60mol N2��g����1.60mol H2��g������Ӧ��һ�������´ﵽƽ��ʱ��NH3�����ʵ���������NH3�����ʵ����뷴Ӧ��ϵ���ܵ����ʵ���֮�ȣ�Ϊ
| 4 |
| 7 |
��5������N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��֪��N2��g��+O2��g���T2NO��g����H=+180.5kJ?mol-1
N2��g��+3H2��g���T2NH3��g����H=-92.4kJ?mol-1
2H2��g��+O2��g���T2H2O��g����H=-483.6kJ?mol-1
����������������һ�����������ˮ�������Ȼ�ѧ����ʽΪ
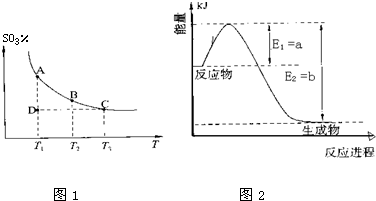
��6���Է�ӦN2O4��g��?2NO2��g�������¶ȷֱ�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ2��ʾ������˵����ȷ����
A��A��C����ķ�Ӧ���ʣ�A��C
B��B��C����������ƽ����Է���������B��C
C��A��C�����������ɫ��A�Cdz
D����״̬B��״̬A�������ü��ȵķ���
��7������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ3��ʾ������YΪCO2��д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ

 ��1��д���豸A��B�����ƣ�A
��1��д���豸A��B�����ƣ�A