��Ŀ����
��9�֣��������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ ���乤���������£�
���乤���������£�
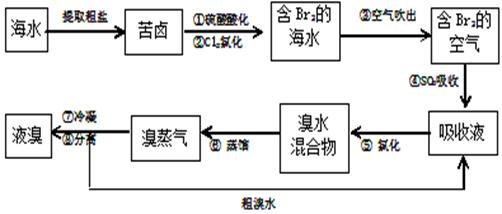
��1���������ڱ���λ�� ���ڣ� �塣
��2������ܵ����ӷ���ʽ�� ��
��3����֪��ķе���58.5�棬�������������У�������¶�Ϊ��Ҫ������80��90�档�� �ȹ�����Ͷ����������� �������ԭ��: ��
�ȹ�����Ͷ����������� �������ԭ��: ��
��4��������������������� �õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ��������������� ������ʱҺ��ӷ������� ����Ͽڡ����¿ڡ����ų���
�õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ��������������� ������ʱҺ��ӷ������� ����Ͽڡ����¿ڡ����ų���
��5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ����� ��
��
 ���乤���������£�
���乤���������£�
��1���������ڱ���λ�� ���ڣ� �塣
��2������ܵ����ӷ���ʽ�� ��
��3����֪��ķе���58.5�棬�������������У�������¶�Ϊ��Ҫ������80��90�档��
 �ȹ�����Ͷ����������� �������ԭ��: ��
�ȹ�����Ͷ����������� �������ԭ��: ����4���������������������
 �õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ��������������� ������ʱҺ��ӷ������� ����Ͽڡ����¿ڡ����ų���
�õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ��������������� ������ʱҺ��ӷ������� ����Ͽڡ����¿ڡ����ų�����5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ�����
 ��
����1������ ��A��2�֣�
��2��Br2+SO2+2H 2O=4H++2Br�D+
2O=4H++2Br�D+  SO42�D��2�֣�
SO42�D��2�֣�
��3���¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ�
�¶ȹ��ͣ��岻����ȫ�����������ʵ͡� ��2�֣�
��4����Һ©�� �¿� ��2�֣�
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��� ������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ������̡���1�֣�
��2��Br2+SO2+2H
 2O=4H++2Br�D+
2O=4H++2Br�D+  SO42�D��2�֣�
SO42�D��2�֣���3���¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ�
�¶ȹ��ͣ��岻����ȫ�����������ʵ͡� ��2�֣�
��4����Һ©�� �¿� ��2�֣�
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ��� ������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2�ĸ������̡���1�֣�
��

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ

 �ᡣ
�ᡣ
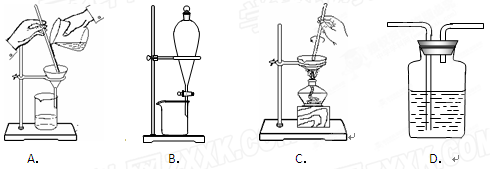
 ϡ������вⶨ��������Ũ�ȴ���1 mol������1�����ƹ��������и�������������������ԭ�� ��
ϡ������вⶨ��������Ũ�ȴ���1 mol������1�����ƹ��������и�������������������ԭ�� �� ��ƿ�мӴ���Һʱ���ζ���δ�ô���Һ��ϴ
��ƿ�мӴ���Һʱ���ζ���δ�ô���Һ��ϴ D.50ml����ƿ
D.50ml����ƿ ����©������ȼ�ճף�����ƽ����Һ©�����߽�ͷ�ιܣ���������
����©������ȼ�ճף�����ƽ����Һ©�����߽�ͷ�ιܣ���������