ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ‘ΎΚΟ―θΨζΚΆ―α―θΨζΉς”Οœ¬Ζœ“Κ÷–NH4+ΡήΉΣΜ·ΈΣN2(g)ΚΆH2O(l)Θ§ Ψ“βΆΦ»γœ¬ΘΚ
Ζ¥”ΠIΘΚNH4+(aq)ΘΪ2O2(g)=NO3Θ≠(aq)ΘΪ2H+(aq)ΘΪH2O(l) ΠΛH1ΘΫa kJΓΛmolΘ≠1
Ζ¥”ΠIIΘΚ5NH4+(aq)ΘΪ3NO3Θ≠(aq)=4N2(g)ΘΪ9H2O(l)ΘΪ2H+(aq) ΠΛH2ΘΫb kJΓΛmolΘ≠1
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. ΝΫ≥ΊΖΔ…ζΒΡΖ¥”Π÷–ΒΣ‘ΣΥΊ÷Μ±Μ―θΜ·
B. ΝΫ≥Ί÷–ΆΕΖ≈ΒΡΖœ“ΚΧεΜΐœύΒ» ±NH4+ΡήΆξ»ΪΉΣΜ·ΈΣN2
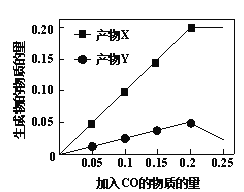
C. ≥ΘΈ¬≥Θ―Ιœ¬Θ§Ζ¥”ΠII÷–…ζ≥…22.4 L N2ΉΣ“ΤΒΡΒγΉ” ΐΈΣ3.75ΓΝ6.02ΓΝ1023
D. 4NH4+(aq)ΘΪ3O2(g)=2N2(g)ΘΪ4H+(aq)ΘΪ6H2O(l) ΠΛHΘΫ![]() (3aΘΪb) kJΓΛmolΘ≠1
(3aΘΪb) kJΓΛmolΘ≠1
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩAΘ°Ζ¥”ΠIΘΚ―θΤχΉς―θΜ·ΦΝΘ§Ζ¥”Π÷–O‘ΣΥΊΒΡΜ·ΚœΦέΚΆN‘ΣΥΊΒΡΜ·ΚœΦέΖΔ…ζ±δΜ·Θ§A¥μΈσΘΜBΘ°Ζ¥”ΠIΘΚ1molNH4+ΉΣΜ·ΈΣ1molNO3-Θ§Ζ¥”ΠIIΘΚ1molNO3-œϊΚΡ![]() NH4+Θ§ΝΫΗωΖ¥”Π÷–œϊΚΡΒΡΖœ“Κ≤ΜΆ§Θ§B¥μΈσΘΜCΘ°≥ΘΈ¬≥Θ―Ιœ¬Θ§ΤχΧεΒΡΡΠΕϊΧεΜΐ≤Μ «22.4L/molΘ§ΈόΖ®ΦΤΥψΒΣΤχΒΡΈο÷ ΒΡΝΩΘ§Υυ“‘ΈόΖ®ΦΤΥψΉΣ“ΤΒΡΒγΉ”ΒΡ ΐΡΩΘ§C¥μΈσΘΜDΘ°ΗυΨίΗ«ΥΙΕ®¬…(3ΓΝI+II)/2Φ¥Ω…ΒΟΒΫ4NH4+(aq)ΘΪ3O2(g)=2N2(g)ΘΪ4H+(aq)ΘΪ6H2O(l) ΠΛHΘΫ
NH4+Θ§ΝΫΗωΖ¥”Π÷–œϊΚΡΒΡΖœ“Κ≤ΜΆ§Θ§B¥μΈσΘΜCΘ°≥ΘΈ¬≥Θ―Ιœ¬Θ§ΤχΧεΒΡΡΠΕϊΧεΜΐ≤Μ «22.4L/molΘ§ΈόΖ®ΦΤΥψΒΣΤχΒΡΈο÷ ΒΡΝΩΘ§Υυ“‘ΈόΖ®ΦΤΥψΉΣ“ΤΒΡΒγΉ”ΒΡ ΐΡΩΘ§C¥μΈσΘΜDΘ°ΗυΨίΗ«ΥΙΕ®¬…(3ΓΝI+II)/2Φ¥Ω…ΒΟΒΫ4NH4+(aq)ΘΪ3O2(g)=2N2(g)ΘΪ4H+(aq)ΘΪ6H2O(l) ΠΛHΘΫ![]() (3aΘΪb) kJΓΛmolΘ≠1Θ§D’ΐ»ΖΓΘ¥πΑΗ―ΓDΓΘ
(3aΘΪb) kJΓΛmolΘ≠1Θ§D’ΐ»ΖΓΘ¥πΑΗ―ΓDΓΘ

 –Γ―ßΩΈΧΟΉς“ΒœΒΝ–¥πΑΗ
–Γ―ßΩΈΧΟΉς“ΒœΒΝ–¥πΑΗ Ϋπ≤© Ω“ΜΒψ»ΪΆ®œΒΝ–¥πΑΗ
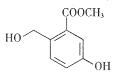
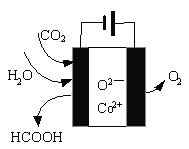
Ϋπ≤© Ω“ΜΒψ»ΪΆ®œΒΝ–¥πΑΗΓΨΧβΡΩΓΩI.”–ΜζΈοΒΡΫαΙΙΩ…”ΟΓΑΦϋœΏ ΫΓ±ΦρΜ·±μ ΨΘ°CH3-CH=CH-CH3Ω…Φρ–¥ΈΣ![]() Θ°”–ΜζΈοXΒΡΦϋœΏ ΫΈΣΘΚ
Θ°”–ΜζΈοXΒΡΦϋœΏ ΫΈΣΘΚ
(1)”–ΜζΈοY «XΒΡΆ§Ζ÷“λΙΙΧεΘ§«“ τ”ΎΖΦœψΧΰΘ§–¥≥ωYΒΡΫαΙΙΦρ Ϋ_______________ΘΜ
(2)Y‘Ύ“ΜΕ®ΧθΦΰœ¬ΖΔ…ζΨέΚœΖ¥”ΠΘ§–¥≥ωΤδΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_______________________ΘΜ
(3)ΕΰΦΉ±Ϋ±ΫΜΖ…œΒΡ“Μδε¥ζΈο”–6÷÷Ά§Ζ÷“λΙΙΧεΘ§’β–©“Μδε¥ζΈο”κ…ζ≥…ΥϋΟ«ΒΡΕ‘”ΠΕΰΦΉ±ΫΒΡ»έΒψΖ÷±πΈΣΘΚ
“Μδε¥ζΕΰΦΉ±Ϋ | 234Γφ | 206Γφ | 213.8Γφ | 204Γφ | 214.5Γφ | 205Γφ |
Ε‘”ΠΕΰΦΉ±Ϋ | 13Γφ | -54Γφ | -27Γφ | -54Γφ | -27Γφ | -54Γφ |
”…“‘…œ ΐΨίΆΤΕœΘΚ
»έΒψΈΣ234ΓφΒΡ“Μδε¥ζΕΰΦΉ±ΫΒΡΫαΙΙΦρ ΫΈΣ____________ΘΜ»έΒψΈΣ-27ΓφΒΡΕΰΦΉ±ΫΒΡΟϊ≥ΤΈΣ____________ΘΜ
II.÷ΈΝΤΦΉ–ΆH1N1ΝςΗ–ΒΡ≥ΘΦϊ“©Έο”–Α¬ΥΨΥϊΈΛΓΔ‘ζΡ«ΟΉΈΛΓΔΫπΗ’““ΑΖΦΑΫπΗ’ΆιΑΖ“‘ΦΑ‘ΛΖά“©Έο»γ÷–“©≤ΡΫπ“χΜ®ΓΔ¥σ«ύ“ΕΒ»ΓΘΤδ÷–ΫπΗ’ΆιΑΖΩ…Α¥œ¬Ν–¬ΖœΏΚœ≥…ΘΚ

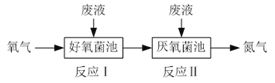
“―÷ΣΒ“ΕϊΥΙΑΔΒ¬ΕζΖ¥”Π(“≤≥ΤΥΪœ©Κœ≥…Ζ¥”Π)»γœ¬Υυ ΨΘΚ

‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)BΒΡΫαΙΙ ΫΈΣ________________(ΧνΦϋœΏ Ϋ)ΓΘ
(2)…œ ωΈο÷ ÷–, τ”ΎΆ§Ζ÷“λΙΙΧεΒΡ «________(ΧνΉ÷ΡΗ)ΓΘ
(3)…œ ωΖ¥”Π÷–, τ”ΎΦ”≥…Ζ¥”ΠΒΡ «________(Χν ΐΉ÷)ΓΘ
ΓΨΧβΡΩΓΩ“―÷ΣΘΚ![]()
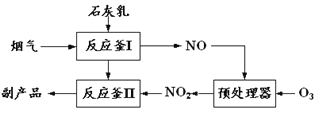
άϊ”Ο»γΆΦΉΑ÷ϔϒΐΕΓ¥ΦΚœ≥…’ΐΕΓ»©![]() œύΙΊ ΐΨί»γ±μΘΚ
œύΙΊ ΐΨί»γ±μΘΚ
Έο÷ | Ζ–Βψ | ΟήΕ» | Υ°÷–»ήΫβ–‘ |
’ΐΕΓ¥Φ |
|
| ΈΔ»ή |
’ΐΕΓ»© |
|
| ΈΔ»ή |
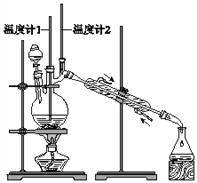
œ¬Ν–ΥΒΖ®÷–Θ§≤Μ’ΐ»ΖΒΡ «![]()
A. ΈΣΖά÷Ι≤ζΈοΫχ“Μ≤Ϋ―θΜ·Θ§”ΠΫΪΥαΜ·ΒΡ![]() »ή“Κ÷πΒΈΦ”»κ’ΐΕΓ¥Φ÷–
»ή“Κ÷πΒΈΦ”»κ’ΐΕΓ¥Φ÷–
B. Β±Έ¬Ε»ΦΤ1 Ψ ΐΈΣ![]() Θ§Έ¬Ε»ΦΤ2 Ψ ΐ‘Ύ
Θ§Έ¬Ε»ΦΤ2 Ψ ΐ‘Ύ![]() Ήσ”“ ±Θ§ ’Φ·≤ζΈο
Ήσ”“ ±Θ§ ’Φ·≤ζΈο
C. Ζ¥”ΠΫα χΘ§ΫΪΝσ≥ωΈοΒΙ»κΖ÷“Κ¬©ΕΖ÷–Θ§Ζ÷»ΞΥ°≤ψΘ§¥÷’ΐΕΓ»©¥”Ζ÷“Κ¬©ΕΖ…œΩΎΒΙ≥ω
D. œρΜώΒΟΒΡ¥÷’ΐΕΓ»©÷–Φ”»κ«β―θΜ·ΡΤΚσ‘Ό’τΝσΘ§Ω…Ϋχ“Μ≤Ϋ≥ΐ»Ξ¥÷≤ζΤΖ÷–ΒΡ’ΐΕΓ¥Φ‘”÷