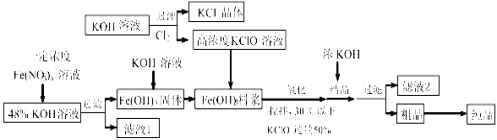
��Ŀ����
����Ŀ��ʵ��������500 mL 0.2 mol/L��Na2SO4��Һ��ʵ����������У�
A������ƽ�ϳ�ȡһ�������������ƹ��壬���������ձ��У�������������ˮʹ����ȫ�ܽⲢ��ȴ�����£�
B�����Ƶõ���ҺС�ĵ�ת�Ƶ�����ƿ�У�
C������������ƿ�м�����ˮ��Һ���̶���1��2 cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����ʹ���̶������У�
D������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ע������ƿ��������
E��������ƿƿ�����������ҡ�ȡ�
�ش��������⣺
��1�������������ȷ˳��Ϊ������ţ� ��
��2������A�У�����ƽ�ϳ�ȡ�����ƹ��������Ϊ g��
��3����ʵ���õ��Ļ��������Ѿ����ձ�����ƽ�������롢���ӣ�������������ȱ�ٵ������� ��
��4���� ��������Һ�����У���ȱ�ٲ���D����ʹ���Ƶ���ҺŨ�� ������ƫ��������ƫ����������Ӱ���������� ����C�м�����ˮʱ�����������˿̶��ߣ���ʹ���Ƶ���ҺŨ�� ������ƫ��������ƫ����������Ӱ��������
��5�����в����У�����ƿ�����߱��Ĺ����� ��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E�����������ܽ��������
���𰸡���1��ABDCE��2��14.2
��3��500mL����ƿ����ͷ�ιܡ�ҩ��
��4��ƫ�ͣ�ƫ�ͣ�5��BCE
�������������������1������һ�����ʵ���Ũ����Һ����Ҫ������������Ϊ���㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ��������֪����ȷ����˳��ΪA��B��D��C��E����2������cV��n�����ʣ�������nM��m�����ʣ�������������֪��m��ԭ�ϣ� = m�����ʣ���������������ɵ����¼���ʽ��m��Na2SO4�� = 0.2��500��10-3��142 g =" 14.2" g����3������Һ��������˳��˼������������������֪���ձ�����ƽ�������롢���ӣ����������⣬����Ҫʹ��500 mL����ƿ����ͷ�ιܡ�ҩ�ף�����ȱ������ƿ�Ĺ��4���� ���ȱ��ϴ�ӻ�ϴ��Һû��ת��������ƿ����V��Ӱ�죬����ʹnƫС������������Һ��![]() ƫ�ͣ����������ˮʱ���������˿̶��ߣ���n��Ӱ�죬������Vƫ����������Һ��
ƫ�ͣ����������ˮʱ���������˿̶��ߣ���n��Ӱ�죬������Vƫ����������Һ��![]() ƫ�ͣ���5��A������ƿ��ר����������һ�����ȷŨ�ȵı���Һ��������A����B������ƿ��������������Һ������������������Һ��������B��ȷ��C��500 mL����ƿֻ�ܲ���500 mL��Һ�壬���������������������Һ�壬C��ȷ��D����Ũ��ҺΪԭ�ϣ�ϡ������һ��Ũ�ȵ�ϡ��Һ����Ҫʹ������ƿ��D����E������ƿ���ܼ��ȣ�ֻ��������20��������ȷ����һ�����һ�����ʵ���Ũ�ȵ���Һ������������n��Ӱ�죬����ʹ��Һ��VƫС��ƫ����������ҺŨ��ƫ��ƫ�ͣ�E��ȷ��
ƫ�ͣ���5��A������ƿ��ר����������һ�����ȷŨ�ȵı���Һ��������A����B������ƿ��������������Һ������������������Һ��������B��ȷ��C��500 mL����ƿֻ�ܲ���500 mL��Һ�壬���������������������Һ�壬C��ȷ��D����Ũ��ҺΪԭ�ϣ�ϡ������һ��Ũ�ȵ�ϡ��Һ����Ҫʹ������ƿ��D����E������ƿ���ܼ��ȣ�ֻ��������20��������ȷ����һ�����һ�����ʵ���Ũ�ȵ���Һ������������n��Ӱ�죬����ʹ��Һ��VƫС��ƫ����������ҺŨ��ƫ��ƫ�ͣ�E��ȷ��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�