��Ŀ����
�����Ϊ2L�������м���1mol N2��6mol H2���п��淴Ӧ�� ������N2�����ʵ���Ϊ0.6mol����
������N2�����ʵ���Ϊ0.6mol����
��1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������____________mol��NH3�����ʵ���������__________mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ��� __________
__________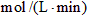 ��
��
��3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���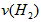 __________
__________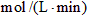 ��
��
��4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���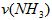 __________
__________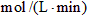 ��
��
��5��ͨ���������㣬����ʲô���֣�______________________
 ������N2�����ʵ���Ϊ0.6mol����
������N2�����ʵ���Ϊ0.6mol������1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������____________mol��NH3�����ʵ���������__________mol��
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���
 __________
__________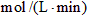 ��
����3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���
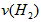 __________
__________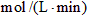 ��
����4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���
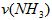 __________
__________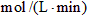 ��
����5��ͨ���������㣬����ʲô���֣�______________________
��1��1.2��0.8����
��2��0.1����
��3��0.3����
��4��0.2������
��5����ѧ��Ӧ�и������ʵķ�Ӧ����֮�ȵȻ�ѧ������֮��
��2��0.1����
��3��0.3����
��4��0.2������
��5����ѧ��Ӧ�и������ʵķ�Ӧ����֮�ȵȻ�ѧ������֮��

��ϰ��ϵ�д�
�����Ŀ
 2SO3��l����2min����O2�����ʵ���Ϊ1.6mol����
2SO3��l����2min����O2�����ʵ���Ϊ1.6mol���� 2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol����
2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol���� 2NH3��
2NH3��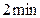 ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol���� 2NH3��
2NH3�� ����N2�����ʵ���Ϊ0.6mol����
����N2�����ʵ���Ϊ0.6mol����