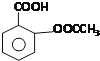
��Ŀ����
����Ŀ����һ������ظ����Σ�KHSO4��2KHSO5��K2SO4��������Ư���������������Ʊ��������£�
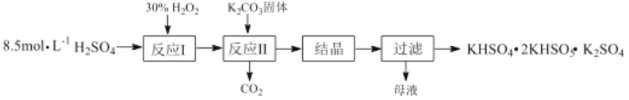
��1����Ӧ��Ļ�ѧ����ʽΪ��H2O2+H2SO4![]() H2SO5+ H2O����H<0����Ӧ����K2CO3��H2SO5��Ӧ����KHSO5�Ļ�ѧ����ʽΪ_____________��
H2SO5+ H2O����H<0����Ӧ����K2CO3��H2SO5��Ӧ����KHSO5�Ļ�ѧ����ʽΪ_____________��
��2������ԭ�ϵ����۱�ֵ[n��H2O2����n��H2SO4��]Ϊ0.4:1����ʵ���������Ͷ�ϱ�Ϊ 0.6:1����ԭ����_________________��
��3����һ������ظ����ο��Դ�����ˮ�е�H2S����֪��25�棬H2S�ĵ��볣��Ka1=1.1��10-7�� Ka2=1.3��10-13���ڵ��볣��ֵ��С��H2Sˮ��Һ��H2S��ƽ��Ũ�Ƚ��Ƶ���H2S�ij�ʼŨ�ȡ�0.090 mol��L-1H2S��Һ��pH=4������Һ��c��S2����=________________��
��4��ȷ��ȡ3.350g��������Ʒ���Ƴ�250mL��Һ��ȡ25.00 mL������ƿ�У�����������ϡ�����������KI��Һ��ҡ�Ⱥ����ڰ�������ַ�Ӧ��������������Һ����0.1000 mol��L-1Na2S2O3����Һ�ζ����յ㣬��������Һ20.00 mL�����㸴��������Ч�ɷ�KHSO5��������������д��������̣�����֪��HSO5- ![]() I2
I2 ![]() S4O62-��________________________��
S4O62-��________________________��
���𰸡�2H2SO5+K2CO3=2KHSO5+CO2��+H2O ������˫��ˮ��Ӧ���ȣ���ʹ˫��ˮ���ַֽ� 1.29��10-13mol��L-1 HSO5-+2I-+H+=SO42-+I2+H2O��I2+2S2O32-=2I-+S4O62-��KHSO5I22Na2S2O3��n��Na2S2O3��=0.1000 mol��L-1��20.00��10-3L=0.002mol��n��I2��=l/2n��Na2S2O3��=l/2��0.002mol=0.00lmol��n��KHSO5��=n��I2��= 0.00lmol��m��KHSO5��= 0.001mol��l 52g/mol=0.152g��![]() ��KHSO5��=��0.152g/3.350g����250mL/25.00mL��100%=45.37%
��KHSO5��=��0.152g/3.350g����250mL/25.00mL��100%=45.37%
��������
���������ͼ��֪��8.5mol/LŨ������30%H2O2��Ӧ��Ũ���Ჿ��ת��Ϊ������H2SO5��ˮ��H2SO5��K2CO3��Ӧ���ɹ�������ء�������̼��ˮ��������Һ���ᾧ�����˵õ���һ������ظ�����KHSO4��2KHSO5��K2SO4��
��1����Ӧ����K2CO3��H2SO5��Ӧ����KHSO5��������̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ2H2SO5+K2CO3=2KHSO5+CO2��+H2O���ʴ�Ϊ��2H2SO5+K2CO3=2KHSO5+CO2��+H2O��
��2���������֪��Ӧ��Ϊ���ȷ�Ӧ����Ӧ�ų�������ʹ��Ӧ�¶����ߣ�H2O2�����ֽ⣬����ʵ��������Ϊ��֤��Ӧ����ѡ������Ͷ�ϱ�Ϊ 0.6:1���ʴ�Ϊ��������˫��ˮ��Ӧ���ȣ���ʹ˫��ˮ���ַֽ⣻
��3��25�棬H2S��ˮ��Һ�������¹�ϵ��Ka1��Ka2=![]() ��
��![]() =
=![]() ���������֪��Һ��c(H+)=1��10-4mol/L��c(H2S)=0.090 mol/L������Һ��c(S2��)=
���������֪��Һ��c(H+)=1��10-4mol/L��c(H2S)=0.090 mol/L������Һ��c(S2��)=![]() =
=![]() ��1.29��10-13 mol/L���ʴ�Ϊ��1.29��10-13 mol/L��
��1.29��10-13 mol/L���ʴ�Ϊ��1.29��10-13 mol/L��
��4���������֪�ⶨ�����з��������·�Ӧ��HSO5-+2I-+H+=SO42-+I2+H2O��I2+2S2O32-=2I-+
S4O62-���ɷ���ʽ�ɵ����¹�ϵ��KHSO5I22Na2S2O3��n��Na2S2O3��=0.1000 mol��L-1��20.00��10-3L=0.002mol�� n��KHSO5��=![]() n��Na2S2O3��=
n��Na2S2O3��=![]() ��0.002mol=0.00lmol��m��KHSO5��= 0.001mol��l 52g/mol=0.152g��
��0.002mol=0.00lmol��m��KHSO5��= 0.001mol��l 52g/mol=0.152g��![]() ��KHSO5��=
��KHSO5��=![]() ��100%=45.37%���ʴ�Ϊ��45.37%��
��100%=45.37%���ʴ�Ϊ��45.37%��

����Ŀ����1����֪��������Ũ��Ϊ0.1mol/L��������Һ��pH���
���� | NaCl | CH3COOK | Na2CO3 | NaClO | NaHCO3 |
pH | 7 | 8.1 | 11.6 | 9.7 | 8.3 |
�ٵ���������ʵ���Ũ�ȵ�NaCl��Һ��NaClO��Һ��Cl-��ClO-���Ӹ�����Cl-_________ClO-��Ũ����ȵ�NaClO��CH3COOK��Һ��:[c(Na+)-c(ClO-)]__________[c(K+)-c(CH3COO-)](����>����<������=��)
��HCO3-��ˮ�ⳣ��Ϊ__________����д������ֵ��������ͬ�¶��£�ͬŨ�ȵ�CH3COOH��H2CO3��HClO��������Һ�ĵ���������СΪ__________
��2�������£���pH=a�Ĵ����м�������NaOHʱ������ԣ����NaOH��pH_____14-a(>��<��=)
��3�������£���֪0.1mol�qL-1һԪ��HA��Һ��![]() =1��10-6��
=1��10-6��
�ٳ����£�0.1mol�qL-1HA��Һ��pH=_____��
��pH=3��HA��pH=11��NaOH��Һ�������Ϻ���Һ��4���������ʵ���Ũ�ȴ�С��ϵ��:_____��
��4�������£�amol/L��ˮ������0.1mol/LH2SO4��Һ��Ϻ���Һ�����ԣ���NH3��H2O�ĵ���ƽ�ⳣ��Kb=_____