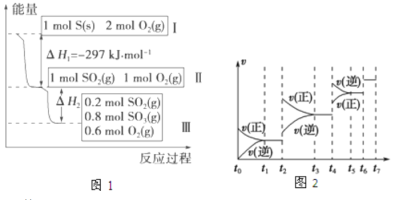
��Ŀ����
����Ŀ������98����ŨH2SO4(��="1.84" g/cm3)���Ƴ�Ũ��Ϊ1.0mol/L��ϡ����250 mL��
��1��������Ҫ������A��Ͳ B�ձ� C������ D��ͷ�ι� ��ȱ�ٵ�����_______________��
��2���뽫���в�����Ű���ȷ˳�����ں����ϣ�
A������Ͳ��ȡŨH2SO4
B�������ߵ�ҡ��
C���ý�ͷ�ιܼ�ˮ���̶�
D��ϴ���ձ���������2-3��
E��ϡ��ŨH2SO4
F����ȴ����Һת������ƿ
�������ȷ˳��Ϊ__________________��
��3�����в����У�����ƿ�����߱��Ĺ�����________(�����)��
A������һ�����ȷŨ�ȵı���Һ
B����ȡһ�������Һ��
C����������ƿ������µ����������Һ��
D��������Һ
E���������Ⱥ��ܽ��������
��4����Ҫ�ش���������
������ŨH2SO4�����Ϊ___________mL��
����ŨH2SO4���ձ��ڱ�����ע��ʢˮ���ձ��У����Ͻ��裬�������������Һ�彦������ʹ���ս��_________ (����ƫ��������ƫ����������Ӱ��������ͬ)��������Ͳϴ��ת�Ƶ�����ƿ��ʹ���ս��_________ ������ʱˮ�Ӷ���õι�������ʹ���_________��
��5����Ũ�������������ˮ���������Һ�����ʵ���Ũ��________9.2 mol/L (������������������������)��
���𰸡���1��250mL����ƿ��2��AEFDCB��3��CDE
��4����13.6 ��ƫ�� ƫ�� ��ƫ�ͣ�5��<
�����������������
��1��������Һ�IJ������裺���ȼ������Ҫ��Ũ���ᣬȻ������Ͳ��ȡ��������ձ���ϡ�ͣ�ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��500mL����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2cmʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ���õ��������У���Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��250mL����ƿ��
��2�����������Ǽ��㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ������ȷ�IJ���˳���ǣ�AEFDCB��
��3��A������ƿ��������һ������ġ�Ũ��ȷ����Һ����A��ȷ��B������ƿֻ��һ���̶ȣ�����ֻ����ȡ�̶������Һ�壬��B��ȷ��C������ƿ�������ƻ��������ƿ������µ����������Һ�壬��C����D������ƿֻ����������һ�����ȷŨ�ȵ���Һ����������������Һ����D����E������ƿ�������ȣ����Բ����������Ⱥ��ܽ�������ʣ���E����ѡCDE��
��4����Ũ��������ʵ���Ũ��c=![]() =
=![]() =18.4mol/L������Ҫ��ŨH2SO4�����ΪVmL������ϡ�Ͷ���CŨVŨ=CϡVϡ��֪��18.4mol/L��VmL=0.5mol/L��500mL��ã�V=13.6mL���ʴ�Ϊ��13.6�����������������Һ�彦�����ᵼ�����ʵ���ʧ����������ҺŨ��ƫ�ͣ�������Ͳϴ��ת�Ƶ�����ƿ���ᵼ������ƫ�࣬��������ҺŨ��ƫ�ߣ�����ʱˮ�Ӷ���õι�������ʹ���ƫ�͡�
=18.4mol/L������Ҫ��ŨH2SO4�����ΪVmL������ϡ�Ͷ���CŨVŨ=CϡVϡ��֪��18.4mol/L��VmL=0.5mol/L��500mL��ã�V=13.6mL���ʴ�Ϊ��13.6�����������������Һ�彦�����ᵼ�����ʵ���ʧ����������ҺŨ��ƫ�ͣ�������Ͳϴ��ת�Ƶ�����ƿ���ᵼ������ƫ�࣬��������ҺŨ��ƫ�ߣ�����ʱˮ�Ӷ���õι�������ʹ���ƫ�͡�

 ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
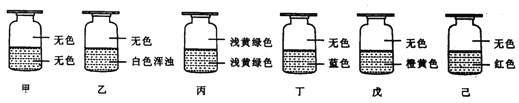
��״Ԫ���źþ�ϵ�д�����Ŀ�����ֹ�������A��B��C��D��E���±��в�ͬ������������ɣ����Ǿ�������ˮ��
������ | Na+ | Al3+ | Fe3+ | Cu2+ | Ba2+ |
������ | OH�� | Cl�� | CO32�� | NO3�� | SO42�� |
�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��A��Һ��C��Һ��Ϻ������ɫ��������ó����м�������ϡHNO3�����������ܽ⣬ʣ���ɫ���壻
��B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������C��Һ��D��Һ��Ϻ������ɫ����������C��Һ��D��Һ��Ϻ�������
��B��Һ��D��Һ��Ϻ�������
����38.4 g CuƬͶ��װ������D��Һ���Թ��У�CuƬ���ܽ⣬�ٵμ�1.6 mol/LϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�
��1���ݴ��ƶ�A�Ļ�ѧʽΪ��A ��
(2)д���������з�����Ӧ�����ӷ���ʽ ��
(3)D��Һ�е���ʯ����Һ�������� ��ԭ���� (�����ӷ���ʽ˵��)��
(4)����������Ҫ��CuƬ��ȫ�ܽ⣬���ټ���ϡH2SO4������� mL��