��Ŀ����
����Ŀ���л�������A��H��ת����ϵ������ʾ��

��ش��������⣺
��1������A��֧����ֻ��һ��������������Է���������65��75֮����1 mol A��ȫȼ������7 mol��������A�Ľṹ��ʽ��____________________________��
��2�����ض�������������A������ʵ�����H2��Ӧ����E��E______(����������������������)˳���칹����Eת��ΪF�Ļ�ѧ����ʽΪ______________________________��
��3��G������Ʒ�Ӧ�ܷų���������Fת��ΪG�ķ�Ӧ������__________________����G����H�Ļ�ѧ����ʽ��__________________��
��4��B��A��һ��ͬ���칹��������һ�ȴ���ֻ��һ��(�����������칹)����B�Ľṹ��ʽΪ________________________��
���𰸡�(CH3)2CHC��CH ������ (CH3)2CHCH===CH2��Br2![]() (CH3)2CHBrCH2Br NaOHˮ��Һ/���� (CH3)2CHCHOHCH2OH��HOCOCH2CH2COOH
(CH3)2CHBrCH2Br NaOHˮ��Һ/���� (CH3)2CHCHOHCH2OH��HOCOCH2CH2COOH![]()
 ��2H2O
��2H2O 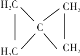
��������
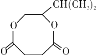
����A��֧����ֻ��һ�������ţ�����Է���������65-75֮�䣬1molA��ȫȼ������7mol������A�ܷ����ӳɷ�Ӧ��˵������̼̼�����ͼ�����A�ķ���ʽΪCxHy��65��12x+7y��75��x��y/4=7������x=5��y=8��A�ķ���ʽΪC5H8������A��֧����ֻ��һ�������ţ���A�Ľṹ��ʽΪCH��CCH��CH3��2�����ض����������£�A������ʵ�����H2��Ӧ����E����E�ṹ��ʽΪCH2=CHCH��CH3��2��B���巢���ӳɷ�Ӧ����F��F�ṹ��ʽΪCH2BrCHBrCH��CH3��2��F����ˮ�ⷴӦ����G��G�ṹ��ʽΪHOCH2CH��OH��CH��CH3��2��G�Ͷ����ᷴӦ����H��H�ṹ��ʽΪ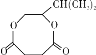 ��
��
��1������A��֧����ֻ��һ�������ţ�����Է���������65-75֮�䣬1molA��ȫȼ������7mol������A�ܷ����ӳɷ�Ӧ��˵������̼̼�����ͼ�����A�ķ���ʽΪCxHy��65��12x+7y��75��x��y/4=7������x=5��y=8��A�ķ���ʽΪC5H8����A�������ӳɵõ�E��E�ܼ�������ӳɣ�����A����̼̼��������A�к���һ��֧������AΪCH��CCH��CH3��2��
��2��A�Ľṹ��ʽΪCH��CCH��CH3��2�����ض����������£�A������ʵ�����H2��Ӧ����E��E�ṹ��ʽΪCH2=CHCH��CH3��2��E������˳���칹��F�ṹ��ʽΪCH2BrCHBrCH��CH3��2����Eת��ΪF�Ļ�ѧ����ʽ��CH2=CHCH��CH3��2+Br2��CH2BrCHBrCH��CH3��2��
��3��G������Ʒ�Ӧ�ܷų����壬˵��G��Ӧ�����ǻ������F��GΪ�����������ȡ����Ӧ���ɴ��ķ�Ӧ������Ϊ��NaOHˮ��Һ/���ȣ�����GΪHOCH2CH��OH��CH��CH3��2 ��Gת��ΪH�Ļ�ѧ����ʽΪ (CH3)2CHCHOHCH2OH��HOCOCH2CH2COOH![]()
 ��2H2O��
��2H2O��
��4��B��A��һ��ͬ���칹�壬����һ�ȴ���ֻ��һ��(�����������칹)����4��̼��Ϊ�Գƽṹ������䲻���Ͷ�Ϊ2�����Ե�֪Ϊ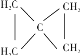 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Li4Ti5O12��LiFePO4��������ӵ�صĵ缫���ϣ���������������Ҫ�ɷ�ΪFeTiO3 �� ����������MgO��SiO2�����ʣ����Ʊ��������������£�
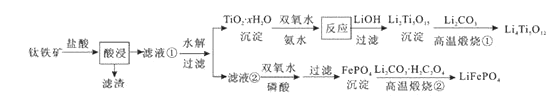
����˵������ȷ���ǣ� ��
A.���������������Ҫ��TiOCl42-��ʽ���ڣ�����Ӧ��Ӧ�����ӷ���ʽ�ɱ�ʾΪ��FeTiO3+4H++4Cl-=Fe2++TiOCl42-+2H2O
B.��Li2Ti5O15��Ti�Ļ��ϼ�Ϊ+4�������й���������ĿΪ3��
C.���������բ�������FePO4�Ʊ�LiFePO4�Ļ�ѧ����ʽ�ɱ�ʾΪ��2FePO4+Li2CO3+H2C2O4![]() 2LiFePO4+H2O��+3CO2��
2LiFePO4+H2O��+3CO2��
D.TiO2 �� xH2O������˫��ˮ����ˮ��Ӧ40min����ʵ�������±���ʾ��
�¶�/ �� | 30 | 35 | 40 | 45 | 50 |
| 92 | 95 | 97 | 93 | 88 |
��֪��40 oCǰ��δ�ﵽƽ��״̬�������¶����ߣ�ת���ʱ��40 oC��H2O2�ֽ�Ӿ磬ת���ʽ���