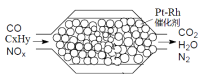
��Ŀ����
����Ŀ�����仯�����ڹ�ҵ����������;����ҵ���������Ϊԭ���Ʊ����ᣬ�������Mg��Fe��Ca��Al��B��O�ȶ���Ԫ�أ�������Ҫ�ɷ�ΪMg2B2O5��H2O��Fe3O4��
��1����Ԫ��λ��Ԫ�����ڱ��еĵ� �壬��ˮ��Һ�г���Fe2+��Fe3+����ʽ���ڣ����� ���ȶ���
��2��������Ϊԭ�Ͽ��Ƶ�NaBH4 ,Bԭ�ӵ��ӻ���ʽΪ ��
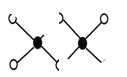
��3����ҵ��ұ���������Ȼ�������Ϊ�Ȼ�������������˫����Al2Cl6�ṹ��ͼ��ʾ��1mol�÷����к� ����λ�����÷��� ����ǡ���ƽ���ͷ��ӡ�
��4��Al����Ϊ�����������壬�侧���ı߳�a=0.405nm����ʽ��ʾAl���ʵ��ܶȣ�____g��cm��3��
������
��1�� �� Fe3+
��2�� sp3
��3��2NA ��
��4�� �ѣ�![]()
��������
�����������1��FeԪ��ԭ�Ӻ��������Ϊ26��λ��Ԫ�����ڱ��еĵ�������Fe3+����3dΪ�����ȶ�״̬�������ϵͣ��Ƚ��ȶ���
��2��NaBH4��Bԭ�Ӽ۲���Ӷ���Ϊ4+![]() =4���ӻ������ĿΪ4�����ӻ���ʽΪsp3��
=4���ӻ������ĿΪ4�����ӻ���ʽΪsp3��
��3��Al2Cl6�ṹ�к�ɫ��ΪAlԭ�ӡ���ɫ��ΪClԭ�ӣ�Alԭ����Clԭ��֮���γ�3�����ۼ���1����λ����Alԭ���ӻ������ĿΪ4��1mol�÷����к�2NA����λ�����÷��Ӳ���ƽ���ͷ��ӣ�
��4��Al����Ϊ�����������壬������Alԭ����ĿΪ8��![]() +6��
+6��![]() =4����������Ϊ4��
=4����������Ϊ4��![]() g���侧�����a=0.405nm�����ܶȦѣ�
g���侧�����a=0.405nm�����ܶȦѣ�![]() ��
��

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�