��Ŀ����
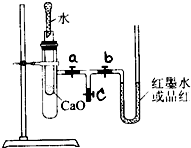 ijУ��ѧ��ȤС���ͬѧ��ͼ��ʾ��ʵ��װ�����Ӻã����л�ѧ��������ת����ʵ��̽����������ǻش��������⣺
ijУ��ѧ��ȤС���ͬѧ��ͼ��ʾ��ʵ��װ�����Ӻã����л�ѧ��������ת����ʵ��̽����������ǻش��������⣺ʵ��ǰ����U���ڼ�������Ʒ����Һ�����īˮ������3��T�������У�ʹU�������ߵ�Һ�洦��ͬһˮƽ�棬�ټн������У�
��1�����ڲ����Թ���ʢ1g�����ƣ�������2mL���ҵ�����ˮ��a��b�����У��ɹ۲쵽U����ĺ�īˮ��α仯��
U����ĺ�īˮ�����ƶ��������Ҳ�Һ��������Һ��
U����ĺ�īˮ�����ƶ��������Ҳ�Һ��������Һ��
����2����������װ�ù۲쵽U���ڳ�����������1���෴��������С�Թ��м���
NH4Cl��NH4NO3�����
NH4Cl��NH4NO3�����
������������������ˮ����3�������ڲ����Թ��з��뼸С��ͭƬ�����õιܵ���10mLϡ���ᣬ�ܷ�õ���1��������
��
��
������ɹ۲쵽��������ͭƬ����������ð������һ������Թܿڸ����к���ɫ������֣�U����ĺ�īˮ�����ƶ�
ͭƬ����������ð������һ������Թܿڸ����к���ɫ������֣�U����ĺ�īˮ�����ƶ�
���������������ԭ����ͭƬ��ϡ���ᷴӦ����NO��NO����������ΪNO2���÷�Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ�
ͭƬ��ϡ���ᷴӦ����NO��NO����������ΪNO2���÷�Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ�
��д���йط�Ӧ�Ļ�ѧ����ʽ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��2NO+O2=2NO2
3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��2NO+O2=2NO2
���ɴ˿�֪����Ӧ������������С��
��
������ڡ�����С�ڡ��������ڡ��������ͭƬ������������ʱ��װ�������ԵIJ��㣬ԭ����NO��NO2��ɢ�������У�����ɻ�����Ⱦ
NO��NO2��ɢ�������У�����ɻ�����Ⱦ
���Ľ��ķ��������Թ��ϼ�һ˫�������õ����ܽ����ɵ����嵼�뵽����������Һ��
���Թ��ϼ�һ˫�������õ����ܽ����ɵ����嵼�뵽����������Һ��
����������1����������ˮ��Ӧ���ȣ���������ѹǿ����
��2��NH4Cl��NH4NO3���������ˮ�����ȵģ���������ѹǿ��С��
��3������ͭ���Ժ����ᷴӦ����������ͭ��һ����������ɫ���壬Ѹ�ٱ���������Ϊ����ɫ�Ķ�����������Ӧ�Ƿ��ȷ�Ӧ������Ӧ��������������������������ʱ����Ӧ�Ƿ��ȵģ���֮�����ȵģ�NO��NO2�����ж����壬Ҫ����β��������
��2��NH4Cl��NH4NO3���������ˮ�����ȵģ���������ѹǿ��С��
��3������ͭ���Ժ����ᷴӦ����������ͭ��һ����������ɫ���壬Ѹ�ٱ���������Ϊ����ɫ�Ķ�����������Ӧ�Ƿ��ȷ�Ӧ������Ӧ��������������������������ʱ����Ӧ�Ƿ��ȵģ���֮�����ȵģ�NO��NO2�����ж����壬Ҫ����β��������
����⣺��1����������ˮ��Ӧ���ȣ����´��Թ�����ѹ��������U����ĺ�īˮ�����ƶ��������Ҳ�Һ��������Һ�棬�ʴ�Ϊ��U����ĺ�īˮ�����ƶ��������Ҳ�Һ��������Һ�棻
��2����������װ�ù۲쵽U���ڳ�����������1���෴������������ˮ�Ӵ����ȵ����ʣ�����Σ��������ˮ���ȣ��ʴ�Ϊ��NH4Cl��NH4NO3����Σ�
��3��ͭƬ�ܺ�ϡ���ᷴӦ����������ͭ��ˮ��һ��������ɫ���壬����3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O����Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ������ɵ�һ����������ɫ���壬Ѹ�ٱ���������Ϊ����ɫ�Ķ�����������2NO+O2=2NO2�������������ж�������Ⱦ�������������β�����մ������������������������գ�����Ӧ��������������������������ʱ����Ӧ�����ȵģ�
�ʴ�Ϊ���ܣ�ͭƬ����������ð������һ������Թܿڸ����к���ɫ������֣�U����ĺ�īˮ�����ƶ���ͭƬ��ϡ���ᷴӦ����NO��NO����������ΪNO2���÷�Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ���3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��2NO+O2=2NO2��С�ڣ�NO��NO2��ɢ�������У�����ɻ�����Ⱦ�����Թ��ϼ�һ˫�������õ����ܽ����ɵ����嵼�뵽����������Һ�У�
��2����������װ�ù۲쵽U���ڳ�����������1���෴������������ˮ�Ӵ����ȵ����ʣ�����Σ��������ˮ���ȣ��ʴ�Ϊ��NH4Cl��NH4NO3����Σ�
��3��ͭƬ�ܺ�ϡ���ᷴӦ����������ͭ��ˮ��һ��������ɫ���壬����3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O����Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ������ɵ�һ����������ɫ���壬Ѹ�ٱ���������Ϊ����ɫ�Ķ�����������2NO+O2=2NO2�������������ж�������Ⱦ�������������β�����մ������������������������գ�����Ӧ��������������������������ʱ����Ӧ�����ȵģ�
�ʴ�Ϊ���ܣ�ͭƬ����������ð������һ������Թܿڸ����к���ɫ������֣�U����ĺ�īˮ�����ƶ���ͭƬ��ϡ���ᷴӦ����NO��NO����������ΪNO2���÷�Ӧ���ȣ�ʹ���Թ�����ѹ������U����ĺ�īˮ�����ƶ���3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O��2NO+O2=2NO2��С�ڣ�NO��NO2��ɢ�������У�����ɻ�����Ⱦ�����Թ��ϼ�һ˫�������õ����ܽ����ɵ����嵼�뵽����������Һ�У�
������������һ���ۺ�֪ʶ��Ŀ������Ƕȹ㣬Ҫ��ѧ�����з����ͽ��������������ѶȽϴ�

��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ