��Ŀ����
a��CH3CH2OH(g)��H2O(g)��4H2(g)��2CO(g) ��H1��+256.6 kJ��mol��1
b��2CH3CH2OH(g)��O2(g)��6H2(g)��4CO(g) ��H2��+27.6 kJ��mol��1
������˵����ȷ����
A. ����a�ķ�Ӧ�¶ȣ��Ҵ���ת��������
B. ��b��֪���Ҵ���ȼ����Ϊ13.8 kJ��mol��1
C. 2H2(g)��O2(g)��2H2O(g) ��H����485.6 kJ��mol��1
D. ��ȡ������������;��b���ĵ���������
��ϰ��ϵ�д�
�����Ŀ




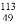 In����������
In����������