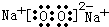��Ŀ����
��1����OH-������������������ͬ�ĵ����������ӽṹʾ��ͼΪ��2��Ҫ֤��ij��Һ����Fe3+�����ܺ���Fe2+����ѵ�ʵ�����˳����
�ټ���������ˮ�ڼ�������KMnO4��Һ�ۼ�������NH4SCN��Һ
A���٢�B���ۢ�C���ۢ�D���٢ڢ�
��3��25��ʱ��2.3g�ƾ���ѪҺ�б�������ȫ�����ų�66.8kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ
��4��������ȫ�����к�MYn�ͺ���ɫ���������M��YΪ������Ԫ�أ�������������֮�͵�����ԭ�ӵ��������������ҿɷ�����ͼ��ת����
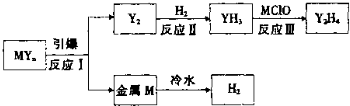
����2L�ܱ������У���ʼͶ��2mol Y2��3.5mol H2������Ӧ���ƽ��ʱ��������������÷�Ӧ��H��0����T1
| �¶� | ƽ��ʱYH3�����ʵ�����mol�� |
| T1 | 1.2 |
| T2 | 1.0 |
�ۺ���ɫ������������Ϊ������Ӧ�����ɵĽ���M���÷�Ӧ�Ļ�ѧ����ʽΪ
��������1�����������Ӻ���9�����Ӻ�10�����ӣ�
��2��Ҫ֤��ij��Һ����Fe3+�����ܺ���Fe2+����Ҫ��������������Ӽ��������ӣ�Ȼ������������۲���Һ�Ƿ��ɺ�ɫ��
��3�������Ȼ�ѧ����ʽ����д������֪��Ӧע�⻯ѧ�������뷴Ӧ�ȵĶ�Ӧ��ϵ�������ʵľۼ�״̬���н��
��4������YH3��֪Y�����5�����ӣ��ٸ��ݡ�M��YΪ������Ԫ�أ�������������֮�͵�����ԭ�ӵ���������������֪M�����1�����ӣ�Ȼ���������ת����ϵ�ƶϳ����������ƣ������¶ȶԸ÷�Ӧ��Ӱ���ж��¶ȹ�ϵ�����ݸ����Ũ�ȱ仯�������Ӧ���ʼ�ƽ�ⳣ�������д����Ӧ�Ļ�ѧ����ʽ��
��2��Ҫ֤��ij��Һ����Fe3+�����ܺ���Fe2+����Ҫ��������������Ӽ��������ӣ�Ȼ������������۲���Һ�Ƿ��ɺ�ɫ��
��3�������Ȼ�ѧ����ʽ����д������֪��Ӧע�⻯ѧ�������뷴Ӧ�ȵĶ�Ӧ��ϵ�������ʵľۼ�״̬���н��
��4������YH3��֪Y�����5�����ӣ��ٸ��ݡ�M��YΪ������Ԫ�أ�������������֮�͵�����ԭ�ӵ���������������֪M�����1�����ӣ�Ȼ���������ת����ϵ�ƶϳ����������ƣ������¶ȶԸ÷�Ӧ��Ӱ���ж��¶ȹ�ϵ�����ݸ����Ũ�ȱ仯�������Ӧ���ʼ�ƽ�ⳣ�������д����Ӧ�Ļ�ѧ����ʽ��
����⣺��1����OH-������Ϊ10��������Ϊ9������9������10�����ӵĵ�������Ϊ�����ӣ���ṹʾ��ͼΪ��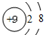 ��
��
�ʴ�Ϊ��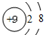 ��
��
��2��֤��ij��Һ����Fe3+�����ܺ���Fe2+��������Һ�м���ۼ�������NH4SCN��Һ���������ӣ���Һ����ʾ��ɫ��֤����Һ�в����������ӣ�Ȼ��������ˮ����Һ��ɺ�ɫ��֤������Һ�д����������ӣ�
�ʴ�Ϊ��C��
��3��2.3g�ƾ������ʵ���Ϊ0.05mol��0.05mol�Ҵ���ѪҺ�б�������ȫ�����ų�66.8kJ��������1mol�Ҵ���ȫ��Ӧ�ų�������Ϊ��66.8kJ��
=1336kJ���Ҵ�ȼ�յ��Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l������H=-1336 kJ?mol -1��
�ʴ�Ϊ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l������H=-1336 kJ?mol-1 ��
��4��M��YΪ������Ԫ�أ�������������֮�͵�����ԭ�ӵ�������������˵������������֮��Ϊ6����Y���⻯��ΪYH3��֪��Y�����5�����ӣ���M�����1�����ӣ��ٸ���ͼʾת����ϵ������MΪ�����ƣ�YΪN��
�ٸ÷�Ӧ��H��0���¶����ߣ������ĺ������С���ɱ������ݿ�֪��T1��T2��������10min��Ӧ�ﵽƽ�⣬��ƽ������v��NH3��=
=0.05mol/��L?min�������ݷ�Ӧ����ʽ��N2��g��+3H2��g���T2NH3�����¶��£��ﵽƽ��״̬ʱ����ֵ�Ũ��Ϊ��c��NH3��=0.5ol/L��c��H2��=
=1mol/L��c��N2��=
=0.75mol/L��ƽ�ⳣ��Ϊ��K=
=0.33��
�ʴ�Ϊ������ 0.05mol/��L?min����0.33��
�ڷ�Ӧ��Ϊ������������Ƶķ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ۺ���ɫ����������Ϊ��������������������Ʒ�Ӧ���������ƺ������÷�Ӧ�Ļ�ѧ����ʽΪ��6Na+Fe2O3�T3Na2O+2Fe��
�ʴ�Ϊ��6Na+Fe2O3�T3Na2O+2Fe��
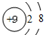 ��
���ʴ�Ϊ��
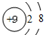 ��
�� ��2��֤��ij��Һ����Fe3+�����ܺ���Fe2+��������Һ�м���ۼ�������NH4SCN��Һ���������ӣ���Һ����ʾ��ɫ��֤����Һ�в����������ӣ�Ȼ��������ˮ����Һ��ɺ�ɫ��֤������Һ�д����������ӣ�
�ʴ�Ϊ��C��
��3��2.3g�ƾ������ʵ���Ϊ0.05mol��0.05mol�Ҵ���ѪҺ�б�������ȫ�����ų�66.8kJ��������1mol�Ҵ���ȫ��Ӧ�ų�������Ϊ��66.8kJ��
| 1mol |
| 0.05mol |
�ʴ�Ϊ��C2H5OH��l��+3O2��g���T2CO2��g��+3H2O��l������H=-1336 kJ?mol-1 ��
��4��M��YΪ������Ԫ�أ�������������֮�͵�����ԭ�ӵ�������������˵������������֮��Ϊ6����Y���⻯��ΪYH3��֪��Y�����5�����ӣ���M�����1�����ӣ��ٸ���ͼʾת����ϵ������MΪ�����ƣ�YΪN��
�ٸ÷�Ӧ��H��0���¶����ߣ������ĺ������С���ɱ������ݿ�֪��T1��T2��������10min��Ӧ�ﵽƽ�⣬��ƽ������v��NH3��=
| ||
| 10min |
| 3.5mol-1.5mol |
| 2L |
| 2mol-0.5mol |
| 2L |
| (0.5)2 |
| (0.75)��(1)3 |
�ʴ�Ϊ������ 0.05mol/��L?min����0.33��
�ڷ�Ӧ��Ϊ������������Ƶķ�Ӧ���䷴Ӧ�Ļ�ѧ����ʽΪ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ʴ�Ϊ��2NH3+NaClO�TN2H4+NaCl+H2O��
�ۺ���ɫ����������Ϊ��������������������Ʒ�Ӧ���������ƺ������÷�Ӧ�Ļ�ѧ����ʽΪ��6Na+Fe2O3�T3Na2O+2Fe��
�ʴ�Ϊ��6Na+Fe2O3�T3Na2O+2Fe��
���������⿼���˻�ѧ��Ӧ���ʵļ��㡢��ѧƽ�ⳣ���ļ��㡢�Ȼ�ѧ����ʽ����д�����������������ӵļ����֪ʶ����Ŀ�ѶȽϴ��漰��֪ʶ��϶࣬ע����ȷ�������ӵļ��鷽�������������ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ