��Ŀ����
9��ijУ��ѧ��ȤС�齫��������·�崦���õ��Ϻ�ɫ����A�����������¿�ͼ��ʾʵ�飬�õ�����ɫ��ҺD����ͼ��ijЩ����������ȥ��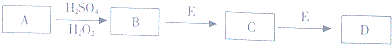
��1��AԪ��λ��Ԫ�����ڱ��е�������IB�壮B��Һ�еĽ��������ӵĵ����Ų�ʽΪ1s22s22p63s23p63d9��
��2��H2O2��������ԭ���ӻ���ʽΪsp3��EΪ���壬��ˮ��Һ�������ԣ�E���ӵ�VSEPRģ�����������ͣ�
��3����D����Һ�м���þ���������ѳ�ȥ����Ĥ����һ��ʱ���þ���������Ϻ�ɫ����������д���÷�Ӧ�����ӷ���ʽ��[Cu��NH3��4]2+Mg+2H2O=Cu+Mg��OH��2+2NH4++2NH3��
���� ����������·�崦���õ��Ϻ�ɫ����AΪCu�������ᡢ�������ⷴӦ�õ�BΪCuSO4��B��E������Ӧ�õ�����ɫ��ҺD����2����EΪ���壬��ˮ��Һ�������ԣ���EΪNH3��CΪCu��OH��2��DΪ[Cu��NH3��4]SO4���ݴ˽��
��� �⣺����������·�崦���õ��Ϻ�ɫ����AΪCu�������ᡢ�������ⷴӦ�õ�BΪCuSO4��B��E������Ӧ�õ�����ɫ��ҺD����2����EΪ���壬��ˮ��Һ�������ԣ���EΪNH3��CΪCu��OH��2��DΪ[Cu��NH3��4]SO4��
��1��AΪCuԪ�أ�λ��Ԫ�����ڱ��е�������IB�壮CuSO4��Һ�еĽ���������ΪCu2+�������Ų�ʽΪ��1s22s22p63s23p63d9��
�ʴ�Ϊ�����ġ�IB��1s22s22p63s23p63d9��
��2��H2O2���ӽṹʽΪH-O-O-H����������ԭ���γ�2���Ҽ�������2�Թµ��Ӷԣ��ӻ������ĿΪ4����ԭ�Ӳ�ȡsp3�ӻ���EΪNH3���۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4��E���ӵ�VSEPRģ���ǣ��������ͣ�
�ʴ�Ϊ��sp3���������ͣ�
��3����[Cu��NH3��4]SO4����Һ�м���þ���������ѳ�ȥ����Ĥ����һ��ʱ���þ���������Ϻ�ɫ�������������Կ���Mg��ͭ���ӷ�Ӧ��þ�����백ˮ��Ӧ���÷�Ӧ�����ӷ���ʽ��[Cu��NH3��4]2+Mg+2H2O=Cu+Mg��OH��2+2NH4++2NH3��
�ʴ�Ϊ��[Cu��NH3��4]2+Mg+2H2O=Cu+Mg��OH��2+2NH4++2NH3��
���� ���⿼�������ƶϣ�ע��������ʵ���ɫ��E��״̬��ˮ��Һ���ʽ����ƶϣ����ض����ʽṹ�Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | ����ʽΪC12H20O2 | |
| B�� | ��ʹ����KMnO4��Һ��ɫ | |
| C�� | �ܷ����ӳɷ�Ӧ�������ܷ���ȡ����Ӧ | |
| D�� | 1mol���л���ˮ��ʱֻ������lmolNaOH |
| A�� | ���³�ѹ�£�11.2LN2��NO�Ļ������������ԭ����ΪNA | |
| B�� | 1mol C8H18�����У����ۼ�����Ϊ25NA | |
| C�� | ��״���£�22.4L���Ậ��nA��HCl���� | |
| D�� | ��0.1molH2O2��ˮ��Һ��MnO2������ã���Ӧ������ת�Ƶĵ�������Ϊ0.2NA |
| A�� | Cl2��SO2��NH3��ˮ��Һ�䶼�ܹ����磬�����Ǿ����ڷǵ���� | |
| B�� | ��Ũ�����м�������Ũ���������������Ƭ����Ƭ�����ۻ� | |
| C�� | �κο��淴Ӧ����ƽ�ⳣ��Խ��Ӧ���ʡ���Ӧ���ת���ʾ�Խ�� | |
| D�� | ��ⱥ��ʳ��ˮ�Ĺ����У�ˮ�ĵ���ƽ�������ƶ� |
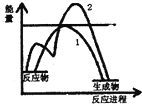 ����CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��Ӧ�й�������ȷ���ǣ�������
����CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��Ӧ�й�������ȷ���ǣ�������| A�� | Ũ�����ڸ÷�Ӧ�����Ž��ͷ�Ӧ��ܺ����ԭ��ת�������� | |
| B�� | �÷�Ӧ������ȥ��Ӧ | |
| C�� | ��ͼ��ʾ���ȷ�Ӧ��ͼ������1��ʾδ�Ӵ����� ������2��ʾ���˴��� | |
| D�� | �÷�ӦΪ���ȷ�Ӧ�������{�¶�����Ӧ�ٶȼ�С���淴Ӧ�ٶȼӿ� |
 ��
��
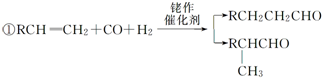
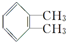 ������Ϊ�ڶ��ױ���
������Ϊ�ڶ��ױ���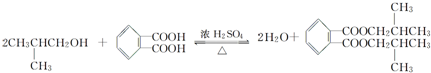 ��
��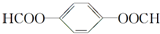 ��X��������2�ֲ�ͬ��ѧ��������ԭ�ӣ�������֮��Ϊ1��2��1mol X��NaOH��Һ���ȷ�Ӧ���������4 mol NaOH��
��X��������2�ֲ�ͬ��ѧ��������ԭ�ӣ�������֮��Ϊ1��2��1mol X��NaOH��Һ���ȷ�Ӧ���������4 mol NaOH��
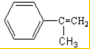
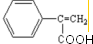
 ����Ӧ��n
����Ӧ��n $\stackrel{һ��������}{��}$
$\stackrel{һ��������}{��}$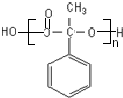 +��n-1��H2O
+��n-1��H2O