��Ŀ����
����Ŀ��A��B��C��D��E��Ϊ�����ڵ�����Ԫ�أ���ԭ��������������A��ԭ�ӵ��Ӳ�������������������B��C���γ����ӻ�����CB2��Dԭ�ӵ�M���������K���������3����
(1)д��A�ļ������ӵĵ���ʽ��________��
(2)E���⻯����C������ȣ��е�ϵ͵���_________(�ѧʽ)��
(3)C��E��ɵĻ�����������ѧ����������______________��
(4)B��D��E��ԭ�Ӱ뾶�ɴ�С��˳������Ϊ_____________(��Ԫ�ط���)��
(5)E������������Ӧ��ˮ���ﻯѧʽ��____________
(6)��D��E����ɻ�����D2E2 ���ڸû������и�ԭ���������ﵽ8�����ȶ��ṹ��д����ṹʽ__________________
(7)д��E��Ԫ�����ڱ��е�λ��____________________________________��
(8)��D����ۺ������Ũ��Һ¶�ÿ����У������ᷢ���仯�����������Ӧ���_____________��
���𰸡�![]() HCl ���Ӽ� S>Cl>F HClO4 Cl-S-S-Cl ��������VII A�� ��ˮ
HCl ���Ӽ� S>Cl>F HClO4 Cl-S-S-Cl ��������VII A�� ��ˮ
��������
A��B��C��D��E��Ϊ�����ڵ�����Ԫ�أ���ԭ��������������A��ԭ�ӵ��Ӳ���������������������AΪHԪ�أ�D��ԭ��M���������K���������3��������M�������Ϊ6����DΪSԪ�أ�E��ԭ���������EΪClԪ�أ�B��C���γ����ӻ�����CB2����C����+2�ۡ�B����-1�ۣ�����ԭ������С������BΪFԪ�ء�CΪMg��
��������������֪AΪHԪ�أ�BΪFԪ�أ�CΪMgԪ�أ�DΪSԪ�أ�EΪClԪ�ء�
(1)A��Ԫ�ط�����H��A�ļ������ӵĵ���ʽ��![]() ��
��
(2)E��Cl��E���⻯��HCl���������ڳ�����Ϊ��̬��C����ΪMg����������Ϊ��̬���������ʵķе�ϵ͵���HCl��
(3)C��E��ɵĻ�����ΪMgCl2���û�����Ϊ���ӻ����Mg2+��2��Cl-ͨ�����Ӽ���ϣ��������������ѧ�������������Ӽ���
(4) BΪFԪ�أ�DΪSԪ�أ�EΪClԪ�أ�ͬһ���ڵ�Ԫ�أ�����ԭ������������ԭ�Ӱ뾶��С��ͬһ����Ԫ�أ�����ԭ������������ԭ�Ӱ뾶�������������Ԫ�ص�ԭ�Ӱ뾶��S��Cl��F��
(5)EΪClԪ�أ�E������������Ӧ��ˮ���ﻯѧʽ��HClO4��
(6)��D��E����ɻ�����D2E2 ��S2Cl2���ڸû������У�2��Sԭ��֮���γ�1�Թ��õ��Ӷԣ�ÿ��Sԭ����1��Clԭ���γ�1�Թ��õ��Ӷԣ������и�ԭ���������ﵽ8�����ȶ��ṹ����ṹʽΪCl-S-S-Cl��
(7) EΪClԪ�أ���������Ų���2��8��7������E��Ԫ�����ڱ��е�λ����λ�ڵ������ڵ�VIIA�塣
(8)DΪS����D����ۺ������Ũ��Һ��ΪŨ���ᣬ�����ʾ�����ˮ�ԣ�¶�ÿ����У������տ����е�ˮ������ʹ��Һ���������ӣ������������Ӧ�����ˮ�ԡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������һ�ַdz���Ҫ�Ľ���Ԫ�أ��ںܶ��������Ź㷺��Ӧ�á����÷���м��ԭ������Ʒλ���̿��Ʊ������̣�Ȼ����е�⣬���Ʊ������̵��¹��գ������̼�ͼ���£�
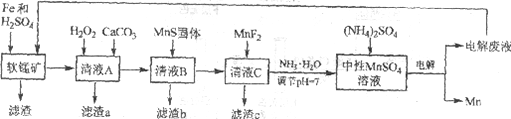
��֪��
i. ��Ʒλ���̿���Ҫ�ɷ���MnO2��Al2O3��Fe2O3��Cu2(OH)2CO3��CaCO3��SiO2�ȡ�
ii. ���ֽ��������������������ʱ��pH
Fe2+ | Fe3+ | Al3+ | Mn2+ | Cu2+ | |
��ʼ������pH | 6.8 | 1.8 | 3.7 | 8.6 | 5.2 |
������ȫ��pH | 8.3 | 2.8 | 4.7 | 10.1 | 6.7 |
iii. ���ֻ�������ܽ��Ի��ܶȻ�(Ksp)
MnF2 | CaS | MnS | FeS | CuS |
����ˮ | ����ˮ | 2.5��10��13 | 6.3��10��18 | 6.3��10��36 |
(1)�о�����������Fe��Fe2+�����Ի�ԭMnO2����������ڵ������£�MnO2��Fe����Ϊspan>Fe3+�����ӷ���ʽ��____________��
(2)��ҺA����H2O2������Ȼ�����CaCO3����Ӧ����Һ��pH��5������a����Ҫ�ɷ����л������NH4Fe3(SO4)2(OH)6��
��H2O2��������____________(�����ӷ���ʽ��ʾ)��
������a�г��˻����������Ҫ�ɷֻ���X����ƽ���ƶ�ԭ�����Ͳ���x��ԭ��____________��
(3)�����ӷ���ʽ��ʾMnS��������ã�____________��
(4)����c�ijɷ���____________��
(5)����ͼ��ʾװ�ã��ö��Ե缫�������MnSO4��Һ�����Ƶý���Mn��������Ӧ�У�
i. Mn2++2e����Mn ii. 2H++2e����H2��
�缫��H2�IJ�������������ǿ��ѣ�Ӱ���Ʒ������
�ٵ��������ĵ缫����ʽ��________________��
����ҺC��Ҫ�ð�ˮ����pH��7��ԭ����____________��
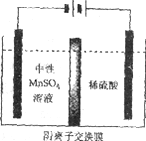
�۵��ʱ������MnSO4��Һ�м���(NH4)2SO4�����ó���������Һ������֮�⣬����___________(��ϵ缫��Ӧʽ�����ӷ���ʽ����)��




