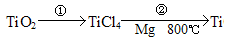��Ŀ����
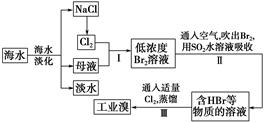
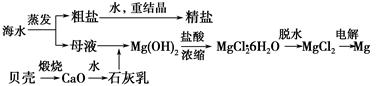
�Ժ���Al2O3��Fe2O3�����ʵĸ�����[��Ҫ�ɷ�ΪFe��CrO2��2]Ϊ��Ҫԭ�������ظ����ƾ��壨 Na2Cr2O7��2H2O������Ҫ�����������£�
��֪���������ڿ������봿����������Na2CrO4��һ�ֺ���ɫ���壬ͬʱ�ͷų�CO2���壬ͬʱA12O3+Na2CO3 2NaAlO2+CO2������ش�
2NaAlO2+CO2������ش�
��1���ڸ�����Fe��CrO2��2�У�Cr�Ļ��ϼ�Ϊ____��
��2������1�ijɷ�Ϊ________������2�ijɷ�Ϊ____��
��3��������l����ϡ�����ܽ�����ҺW���������Һ�н������ӵķ����� ��
��4���������е�Al2O3�����ڹ�ҵ�Ͽ�����ұ�������û�ѧ����ʽΪ
��5�������йع��ұ�����CrO42���ķ�ˮҪ����ѧ������ʹ��Ũ�Ƚ���5��0��10��7 mo1/L���²����ŷš���CrO42���ķ�ˮ����ͨ�����������ַ�����
�ٳ���������������Ա�������BaCrO4���� ���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
���ټ�������������δ��������Ba2+����������Ա��κ�ķ�ˮ��Ba2+��Ũ��Ӧ��С�� mol/L��������ˮ�������ܴﵽ�����ŷű���
�ڻ�ԭ����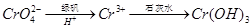 ��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
��д������������ CrO42�����̷�����Һ�з�Ӧ�����ӷ���ʽ ��
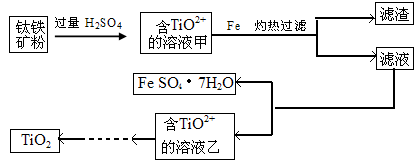
��6��ij��Ч��ˮ������K2FeO4�õ��ģ���ҵ������ҺW��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1��+3��1�֣�
��2��Fe2O3 Al��OH��3����1�֣�������������Ҳ�ɣ�
��3��ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+������������Ҳ�ɣ���2�֣�
��4��2 Al2O3 4Al + 3O2����1�֣�
4Al + 3O2����1�֣�
��5����2��4��10��4��2�֣���CrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O��3�֣�
��6��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O��3�֣�
���������������1���ڸ�����Fe��CrO2��2�У�Fe�Ļ��ϼ�Ϊ+2������Cr�Ļ��ϼ�Ϊ+3��
��2�������ʵĸ��������ա�����ˮ��ֻ��������������ˮ����Ϊ������ȥ����������1�ijɷ���Fe2O3����ʱ��ҺΪNa2CrO4�� NaAlO2�Ļ��Һ��ͨ��CO2������NaAlO2��Ӧ����Al��OH��3����������2�ijɷ���Al��OH��3��
��3��������l����ϡ�����ܽ�����ҺW Fe2��SO4��3������Fe3+�ķ�����ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ��죬֤����Fe3+
��4����ҵ���õ�������������ķ������õ�������ѧ����ʽΪ2 Al2O3 4Al + 3O2����
4Al + 3O2����
��5����Ksp��BaCrO4��=1��2��10��10��c��CrO42����<=5��0��10��7 mo1/L,����c��Ba2+��>=1��2��10��10/5��0��10��7=2��4��10��4 mo1/L, ������������ CrO42�����̷�FeSO4?7 H2O��Ӧ��CrO42�������������̷�����ԭ���������ӷ���ʽΪCrO42����3Fe2+��8H+��Cr3+��3Fe3+��4H2O
��6��W ΪFe2��SO4��3������ҺFe2��SO4��3��������غ���������Ϊԭ���Ʊ�K2FeO4���÷�Ӧ�Ļ�ѧ����ʽ��Fe2��SO4��3 + 3KClO + 10KOH = 2K2FeO4 + 3K2SO4 + 3KCl + 5H2O
���㣺���黯�����л��ϼ۵��жϡ����ӵļ��顢����ұ�����ܶȻ����йؼ��㡢������ԭ��Ӧ������ж�

����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�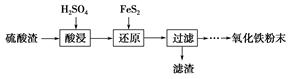
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
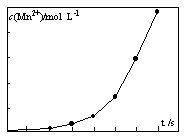
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
ʵ���ѡ�õ��Լ��У�ϡ���ᡢBa(NO3)2��Һ������KMnO4��Һ��NaOH��Һ��Ҫ���Ʊ������в������ж����塣
������ɡ����ˡ������Һģ���Ʊ���������ʵ�鲽�裺
a��������________________________________________________________��
b��������__________________________________________________________��
c�����룬ϴ�ӣ�
d����ɣ���ĥ��
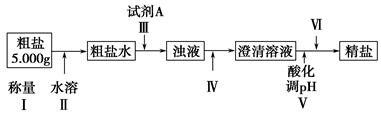
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�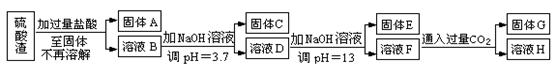
�����ϵ�֪��
| �������� | �ܶȻ�(Ksp) | pHֵ | |
| ��ʼ���� | ��ȫ���� | ||
| Mg(OH)2 | 5.6��10��12 | 9.3 | 10.8 |
| Fe(OH)3 | 2.8��10��16 | 2.7 | 3.7 |
| Al(OH)3 | 1.3��10��33 | 3.7 | 4.7 |
��ش��������⣺
��1��д������A�Ļ�ѧʽΪ ��
��2����Ҫ�ⶨ��Һ��pH�Ƿ�ﵽ3.7������ʵ����Ʒ�п�ѡ�õ��� ��
A��ʯ����Һ B���㷺pH��ֽ C������pH��ֽ D��pH��
��3������������ӷ�Ӧ����ʽ
����ҺD���ɹ���E �� ����ҺF���ɹ���G ��
��4��Ҫ������C������E����G��ת��Ϊ��Ӧ���ȶ����������е�ʵ�����Ϊ ��
��5������������Һ����ı仯���������ҺH��c��Mg2����= ��