��Ŀ����
��6�֣��۱������2С�⣬ÿλͬѧֻ��ѡ����С�⡣�����ⶼ�������ڣ�1��С��Ʒ֡���
��1��A��B��C��Ϊ������Ԫ�أ�����AԪ�صĵ�������Ȼ����������壬BԪ��ԭ�ӵĴ���������Ϊ���������������ڲ������֮�ͣ�CԪ����BԪ��λ��ͬһ���壬�ҿ��γ�BC2�ͻ����
��д��A��C��Ԫ�ط��ţ�A ��C ��
��д��BԪ������������Ӧˮ����Ļ�ѧʽ ��
��2��ij��������A���仯����֮�������µ�ת����ϵ��
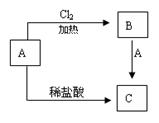
��д��A��C�Ļ�ѧʽ��A ��C__ ��
��д��B��C�����ӷ���ʽ ��
��1��A��B��C��Ϊ������Ԫ�أ�����AԪ�صĵ�������Ȼ����������壬BԪ��ԭ�ӵĴ���������Ϊ���������������ڲ������֮�ͣ�CԪ����BԪ��λ��ͬһ���壬�ҿ��γ�BC2�ͻ����

��д��A��C��Ԫ�ط��ţ�A ��C ��
��д��BԪ������������Ӧˮ����Ļ�ѧʽ ��
��2��ij��������A���仯����֮�������µ�ת����ϵ��

��д��A��C�Ļ�ѧʽ��A ��C__ ��
��д��B��C�����ӷ���ʽ ��

��

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
�����Ŀ
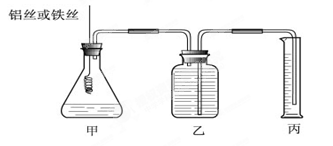

 ��1��
��1��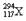 �������
�����й���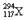 ��˵��������ȷ����
��˵��������ȷ����
 Ҫϡ��Ϊ0.15mol/L��ϡ��Һ480mL����Ҫ���������ձ�������������Ͳ����ͷ�ιܡ�����Ҫ_________________����ȡŨ��Һ�������______mL�������ù������������ʱ���ӿ̶��ߣ������������ҺŨ��________���ƫ�ߡ�����ƫ�͡�����Ӱ�족,��ͬ�������ʵ���������ϴ����Ͳ�����������ҺŨ��_______��
Ҫϡ��Ϊ0.15mol/L��ϡ��Һ480mL����Ҫ���������ձ�������������Ͳ����ͷ�ιܡ�����Ҫ_________________����ȡŨ��Һ�������______mL�������ù������������ʱ���ӿ̶��ߣ������������ҺŨ��________���ƫ�ߡ�����ƫ�͡�����Ӱ�족,��ͬ�������ʵ���������ϴ����Ͳ�����������ҺŨ��_______��