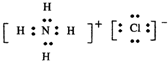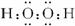��Ŀ����
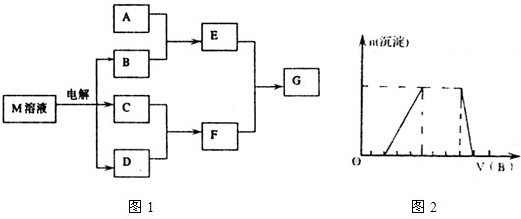
���ʵ�ת����ϵ��ͼ1��ʾ���еķ�Ӧ������ˮ��Һ�н��У������м�Ϊ����ɫ���廯�����Ϊ�������ʣ�GΪ�ᣬ����G��Ũ��Һ�з����ۻ����������������ֱ�պȡ������A��G��Ũ��Һ��ʹ���ǽӽ����д����������ɣ���ش��������⣺

��ش�Щ�����⣺
��1��д���ĵ���ʽ��
 ��
��
��2��д����Ӧ��Ļ�ѧ����ʽ
��3��A����ˮ�õ��õ���ҺX����25���£���a mol?L-1��X��b mol?L-1��G��Һ�������ϣ���Һ�����ԣ���������Һ������Ũ���ɴ�С��˳��Ϊ
��4����ҵ�ϲ��õ�һ����ˮ���������ǣ�������ˮ��pH��5.0-6.0֮�䣬ͨ����ͼ2װ�ô�����ˮ��
�ٵ������������������缫��Ӧ������һ����Ӧ����һ����ɫ���壬�������ĵ缫��Ӧʽ�ֱ��ǣ�
��
��Ϊ��ʹ��ȼ�ϵ�س�ʱ���ȶ����У���صĵ�������Ӧ�����ȶ�����ع���ʱ�����в���A���ʲμ�ѭ��������ͼ����A���ʵĻ�ѧʽ��

��ش�Щ�����⣺
��1��д���ĵ���ʽ��


��2��д����Ӧ��Ļ�ѧ����ʽ
4NH3+5O2
4NO+6H2O
| ||
| �� |
4NH3+5O2
4NO+6H2O
��
| ||
| �� |
��3��A����ˮ�õ��õ���ҺX����25���£���a mol?L-1��X��b mol?L-1��G��Һ�������ϣ���Һ�����ԣ���������Һ������Ũ���ɴ�С��˳��Ϊ
c��NO3-��=c��NH4+����c��OH-��=c��H+��
c��NO3-��=c��NH4+����c��OH-��=c��H+��
�ú�a��b�Ĵ���ʽ��ʾ���û����Һ��X�ĵ���ƽ�ⳣ��k=
| b��10-7 |
| a-b |
k=
��| b��10-7 |
| a-b |
��4����ҵ�ϲ��õ�һ����ˮ���������ǣ�������ˮ��pH��5.0-6.0֮�䣬ͨ����ͼ2װ�ô�����ˮ��
�ٵ������������������缫��Ӧ������һ����Ӧ����һ����ɫ���壬�������ĵ缫��Ӧʽ�ֱ��ǣ�
��
Al-3e-=Al3+
Al-3e-=Al3+
����4OH--4e-=2H2O+O2��
4OH--4e-=2H2O+O2��
����Ϊ��ʹ��ȼ�ϵ�س�ʱ���ȶ����У���صĵ�������Ӧ�����ȶ�����ع���ʱ�����в���A���ʲμ�ѭ��������ͼ����A���ʵĻ�ѧʽ��
CO2
CO2
����������Ϊ����ɫ���廯���ӦΪNa2O2���������������ֱ�պȡ������A��G��Ũ��Һ��ʹ���ǽӽ����д����������ɣ�˵��AӦΪNH3����CΪNO��BΪH2O��EΪNO2��GΪHNO3������G��Ũ��Һ�з����ۻ���ӦΪAl������FΪNaAlO2���������ʵ����ʽ����Ŀ��Ҫ��ɽ����⣮
����⣺��Ϊ����ɫ���廯���ӦΪNa2O2���������������ֱ�պȡ������A��G��Ũ��Һ��ʹ���ǽӽ����д����������ɣ�˵��AӦΪNH3����CΪNO��BΪH2O��EΪNO2��GΪHNO3������G��Ũ��Һ�з����ۻ���ӦΪAl������FΪNaAlO2��
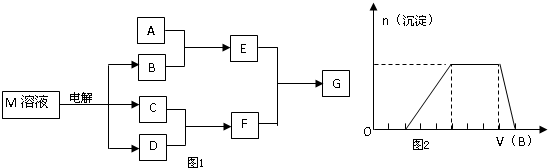
��1�������Ϸ�����֪��ΪNa2O2��Ϊ���ӻ��������ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2����Ӧ��Ϊ�����Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��3��AΪNH3��GΪHNO3����Һ��Ϻ���ڵ���غ㣺c��NO3-��+c��OH-��=c��NH4+��+c��H+������pH=7����c��OH-��=c��H+����c��NO3-��=c��NH4+����������Һ��c��OH-����c��H+����С����c��NO3-��=c��NH4+����c��OH-��=c��H+����
��Ӧ��ʣ��n��NH3?H2O��=��a-b��mol��n��NH4+��=n��NO3-��=bmol��c��OH-��=10-7mol/L��
��k=
=
=
���ٶ���Ϻ���Һ�����ΪV����
�ʴ�Ϊ��c��NO3-��=c��NH4+����c��OH-��=c��H+����k=
��
��4���ٵ�������ΪAl�������������缫��Ӧ������һ����Ӧ����һ����ɫ���壬Ӧ����O2��
�缫����ʽ�ֱ�ΪAl-3e-=Al3+��4OH--4e-=2H2O+O2����
�ʴ�Ϊ��Al-3e-=Al3+�� 4OH--4e-=2H2O+O2����
�ڸ����缫����ʽΪCH4-8e-+4CO32-=5CO2+2H2O������Ϊ2O2+4CO2+8e-=4CO32-����ѭ�����õ�ΪCO2��
�ʴ�Ϊ��CO2��
��1�������Ϸ�����֪��ΪNa2O2��Ϊ���ӻ��������ʽΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2����Ӧ��Ϊ�����Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ4NH3+5O2
| ||
| �� |
�ʴ�Ϊ��4NH3+5O2
| ||
| �� |
��3��AΪNH3��GΪHNO3����Һ��Ϻ���ڵ���غ㣺c��NO3-��+c��OH-��=c��NH4+��+c��H+������pH=7����c��OH-��=c��H+����c��NO3-��=c��NH4+����������Һ��c��OH-����c��H+����С����c��NO3-��=c��NH4+����c��OH-��=c��H+����
��Ӧ��ʣ��n��NH3?H2O��=��a-b��mol��n��NH4+��=n��NO3-��=bmol��c��OH-��=10-7mol/L��
��k=
| c(NH4+)c(OH-) |
| c(NH3?H2O) |
| ||
|
| b��10-7 |
| a-b |
�ʴ�Ϊ��c��NO3-��=c��NH4+����c��OH-��=c��H+����k=
| b��10-7 |
| a-b |
��4���ٵ�������ΪAl�������������缫��Ӧ������һ����Ӧ����һ����ɫ���壬Ӧ����O2��
�缫����ʽ�ֱ�ΪAl-3e-=Al3+��4OH--4e-=2H2O+O2����
�ʴ�Ϊ��Al-3e-=Al3+�� 4OH--4e-=2H2O+O2����
�ڸ����缫����ʽΪCH4-8e-+4CO32-=5CO2+2H2O������Ϊ2O2+4CO2+8e-=4CO32-����ѭ�����õ�ΪCO2��
�ʴ�Ϊ��CO2��
����������Ŀ��������ƶ�Ϊ���壬�ۺϿ���������ʵĵ����Լ��绯ѧ֪ʶ���״���Ϊ��3����ע�����ƽ�ⳣ���ļ��㣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ