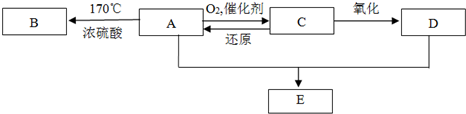
��Ŀ����
ij�л���A��C��H��O����Ԫ����ɣ�15g A��ȫȼ�տ�����22g CO2��9gH2O��������1�����л�������ʽ______��
��2�������ϸ����ʽ��A���ʿ��ܲ�ֹһ�֣�������֮��Ĺ�ϵ______������ţ�
A��һ����Ϊͬϵ�� B��һ����Ϊͬ���칹��
C����������������ȫȼ�պ�������ͬ D�������ʵ�����������ȫȼ�պ�������ͬ
��3����Aֻ��һ�ֹ���������̼������Һ��Ӧ������ų�����÷�Ӧ�Ļ�ѧ����ʽΪ��______��
��4����A�Ǿ���ˮ����ζ��Һ�壬�ɷ���ˮ�ⷴӦ����A�Ľṹ��ʽΪ______��
��5����A����Է�������Ϊ180���ܷ���������Ӧ��Ҳ�ܷ���������Ӧ����A�Ľṹ��ʽΪ______��
���𰸡���������1�����������غ��֪������22g �Ķ�����̼��CԪ��������Ϊ������������CԪ������������9g��ˮ��HԪ��������Ϊ������������HԪ����������������C��H����Ԫ�������͵IJ�Ϊ��Ԫ�ص�������Ȼ��������Ե����ʵ�������̼���⡢��Ԫ�ص����ʵ���֮�ȿ��Ʋ�ʵ��ʽ�����ʽ����
��2��A���ṹ���ƣ�������������1���������ɸ����ŵĻ����ﻥ��Ϊͬϵ��ͬϵ�
B������ͬ����ʽ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻
C�����ʽ��ͬ��������ȣ�����C��H��O������ͬ����ȫȼ�պ�������ͬ��
D�����ʽ��ͬ������ʽ��һ����ͬ�������ʵ�������ȫȼ�պ�������һ����ͬ��
��3��ֻ��һ�ֹ���������̼������Һ��Ӧ������ų��������Ȼ���
��4��������ˮ����ζ��Һ�壬�ɷ���ˮ�ⷴӦ��������Ӧ�������봼��
��5����Է�������Ϊ180�����ʵ��ʽ�������ʽ���ܷ���������Ӧ������ȩ����Ҳ�ܷ���������Ӧ�����Ȼ����ǻ���������ݷ���ʽȷ������
����⣺��1�����������غ㶨�ɵã�15g A������������CԪ������Ϊ��22g×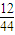 =6g��
=6g��
������������HԪ������Ϊ��9g× =1g��
=1g��
������������OԪ������Ϊ��15g-6g-1g=8g��
n��C����n��H����n��O��=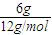 ��
��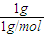 ��
�� =1��2��1�����Ի������ʵ��ʽ�����ʽ����CH2O��
=1��2��1�����Ի������ʵ��ʽ�����ʽ����CH2O��
�ʴ�Ϊ��CH2O��
��2��A�����ʽ��ͬ����һ����ͬϵ��籽����Ȳ��ͬϵ�����ʽҲ��һ����ͬ������������飬��A����
B�����ʽ��ͬ����һ����ͬ���칹�壬�籽����Ȳ����B����
C�����ʽ��ͬ��������ȣ�����C��H��O������ͬ����ȫȼ�պ�������ͬ����C��ȷ��
D�����ʽ��ͬ������ʽ��һ����ͬ�������ʵ�������ȫȼ�պ�������һ����ͬ����1mol�ı�����Ȳ����D����
�ʴ�Ϊ��C��
��3��ֻ��һ�ֹ���������̼������Һ��Ӧ������ų��������Ȼ������ʽΪCH2O��������һ���Ȼ�������ʽΪC2H4O2��
Ϊ�������ᣬ����AΪ���ᣬ�ṹ��ʽΪCH3COOH����Na2CO3��Ӧ�ķ���ʽΪ2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
�ʴ�Ϊ��2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
��4��������ˮ����ζ��Һ�壬�ɷ���ˮ�ⷴӦ��������Ӧ�������봼��������һ������������ʽΪC2H4O2��Ϊ����һԪ��������AΪ����������ṹ��ʽΪHCOOCH3��
�ʴ�Ϊ��HCOOCH3��
��5�������ʽΪ��CH2O��n����Է�������Ϊ180������30n=180�����n=6������ʽΪC6H12O6�������Ͷ�Ϊ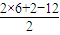 =1���ܷ���������Ӧ��Ҳ�ܷ���������Ӧ������Ϊ�����ǣ��ṹ��ʽΪCH2OH��CHOH��4CHO���ʴ�Ϊ��CH2OH��CHOH��4CHO��
=1���ܷ���������Ӧ��Ҳ�ܷ���������Ӧ������Ϊ�����ǣ��ṹ��ʽΪCH2OH��CHOH��4CHO���ʴ�Ϊ��CH2OH��CHOH��4CHO��
���������⿼���л�������ʡ��ṹ�����ʽȷ�����ѶȲ���ͨ�����������ո������ʵ����ʣ�����Ϥ�л������ʽ�ļ��㷽�������
��2��A���ṹ���ƣ�������������1���������ɸ����ŵĻ����ﻥ��Ϊͬϵ��ͬϵ�
B������ͬ����ʽ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻
C�����ʽ��ͬ��������ȣ�����C��H��O������ͬ����ȫȼ�պ�������ͬ��
D�����ʽ��ͬ������ʽ��һ����ͬ�������ʵ�������ȫȼ�պ�������һ����ͬ��
��3��ֻ��һ�ֹ���������̼������Һ��Ӧ������ų��������Ȼ���
��4��������ˮ����ζ��Һ�壬�ɷ���ˮ�ⷴӦ��������Ӧ�������봼��
��5����Է�������Ϊ180�����ʵ��ʽ�������ʽ���ܷ���������Ӧ������ȩ����Ҳ�ܷ���������Ӧ�����Ȼ����ǻ���������ݷ���ʽȷ������
����⣺��1�����������غ㶨�ɵã�15g A������������CԪ������Ϊ��22g×
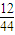 =6g��
=6g��������������HԪ������Ϊ��9g×
 =1g��
=1g��������������OԪ������Ϊ��15g-6g-1g=8g��
n��C����n��H����n��O��=
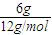 ��
��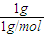 ��
�� =1��2��1�����Ի������ʵ��ʽ�����ʽ����CH2O��
=1��2��1�����Ի������ʵ��ʽ�����ʽ����CH2O���ʴ�Ϊ��CH2O��
��2��A�����ʽ��ͬ����һ����ͬϵ��籽����Ȳ��ͬϵ�����ʽҲ��һ����ͬ������������飬��A����
B�����ʽ��ͬ����һ����ͬ���칹�壬�籽����Ȳ����B����
C�����ʽ��ͬ��������ȣ�����C��H��O������ͬ����ȫȼ�պ�������ͬ����C��ȷ��
D�����ʽ��ͬ������ʽ��һ����ͬ�������ʵ�������ȫȼ�պ�������һ����ͬ����1mol�ı�����Ȳ����D����
�ʴ�Ϊ��C��
��3��ֻ��һ�ֹ���������̼������Һ��Ӧ������ų��������Ȼ������ʽΪCH2O��������һ���Ȼ�������ʽΪC2H4O2��
Ϊ�������ᣬ����AΪ���ᣬ�ṹ��ʽΪCH3COOH����Na2CO3��Ӧ�ķ���ʽΪ2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
�ʴ�Ϊ��2CH3COOH+Na2CO3��2CH3COONa+CO2��+H2O��
��4��������ˮ����ζ��Һ�壬�ɷ���ˮ�ⷴӦ��������Ӧ�������봼��������һ������������ʽΪC2H4O2��Ϊ����һԪ��������AΪ����������ṹ��ʽΪHCOOCH3��
�ʴ�Ϊ��HCOOCH3��
��5�������ʽΪ��CH2O��n����Է�������Ϊ180������30n=180�����n=6������ʽΪC6H12O6�������Ͷ�Ϊ
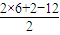 =1���ܷ���������Ӧ��Ҳ�ܷ���������Ӧ������Ϊ�����ǣ��ṹ��ʽΪCH2OH��CHOH��4CHO���ʴ�Ϊ��CH2OH��CHOH��4CHO��
=1���ܷ���������Ӧ��Ҳ�ܷ���������Ӧ������Ϊ�����ǣ��ṹ��ʽΪCH2OH��CHOH��4CHO���ʴ�Ϊ��CH2OH��CHOH��4CHO�����������⿼���л�������ʡ��ṹ�����ʽȷ�����ѶȲ���ͨ�����������ո������ʵ����ʣ�����Ϥ�л������ʽ�ļ��㷽�������

��ϰ��ϵ�д�
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д�
�����Ŀ