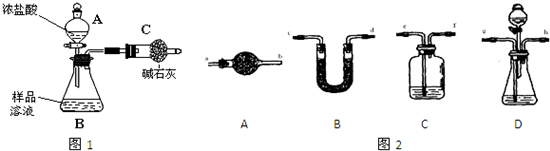
��Ŀ����
���ͼ��ѡ�ñ�Ҫ��װ�ý��е�ⱥ��ʳ��ˮ��ʵ�飬Ҫ��ⶨ�����������������������������
��1��A�������ĵ缫��Ӧʽ��
��2�������������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ��A��
��3��֤����������Cl2��ʵ��������
��4����֪�����ò�����H2�����Ϊ44.8mL���Ѿ�����ɱ�״������������Һ�����Ϊ50mL����ʱ��Һ��NaOH�����ʵ���Ũ��Ϊ��

��1��A�������ĵ缫��Ӧʽ��
2H++2e-=H2��
2H++2e-=H2��
��B�������ĵ缫��Ӧʽ��2Cl--2e-=Cl2��
2Cl--2e-=Cl2��
����2�������������ʵ��װ��ʱ�����ӿڵ���ȷ����˳��Ϊ��A��
G
G
��F
F
��H
H
��B��D
D
��E
E
��C
C
����3��֤����������Cl2��ʵ��������
���۵⻯����Һ����ɫ
���۵⻯����Һ����ɫ
����4����֪�����ò�����H2�����Ϊ44.8mL���Ѿ�����ɱ�״������������Һ�����Ϊ50mL����ʱ��Һ��NaOH�����ʵ���Ũ��Ϊ��
0.08mol/L
0.08mol/L
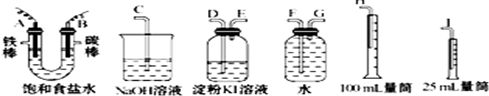
����������1�����ж����������ٸ������ӵķŵ�˳����д��Ӧ�ĵ缫��Ӧʽ��
��2������ʵ��Ŀ�ġ��������������ʵ��װ��˳��
��3������������������ʷ�����
��4�������������������ƵĹ�ϵʽ�����������Ƶ����ʵ������ٸ������ʵ���Ũ�ȹ�ʽ������Ũ�ȣ�
��2������ʵ��Ŀ�ġ��������������ʵ��װ��˳��
��3������������������ʷ�����
��4�������������������ƵĹ�ϵʽ�����������Ƶ����ʵ������ٸ������ʵ���Ũ�ȹ�ʽ������Ũ�ȣ�
����⣺��1�����ǻ��ý�����������������ڵ����������£���ʧ���ӵ���������������ʧ���ӵ����������Ե��ʱ���ܵõ�����������ֻ����������̼���Ƕ��Ե缫����������
��ⱥ��ʳ��ˮʱ�������ӵõ����������������ӣ�������������A���������ӵõ�������������������ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2����
������ʧ���������������������ӣ������������ϼ�B����������ʧ������������������������Ӧ���缫��ӦʽΪ��2Cl--2e-=Cl2����
�ʴ�Ϊ��2H++2e-=H2����2Cl--2e-=Cl2����
��2��A���ϲ�����������������ˮ���ռ���������Ϊ�������ܶ�С��ˮ�ģ�����Ҫ����������ˮ���ռ������̵���Ϊ�����ܣ�������Ϊ��ˮ�ܣ���������˳��ΪA��G��F����Ϊ�ռ��������������25mL������Ҫ��100mL����Ͳ�ռ�ˮ������F����H��
B���ϲ�����������������Ҫ������������ͨ�����۵⻯����Һ���飬������ǿ�����ԣ��ܺ͵⻯�ط�Ӧ���ɵ⣬�������۱���ɫ���������ܶ�С�ڵ⻯����Һ���ܶȣ����Գ�����Ϊ�����ܣ��̵���Ϊ�����ܣ������ж���ֱ���ſ���Ⱦ�������������ͼӦ���������ʣ����Կ��ü�Һ���ն������������������˳��ΪB��D��E��C����
�ʴ�Ϊ��A��G��F��H��B��D��E��C����
��3����ΪCl2+2KI=I2+2KCl���������۱���ɫ�����Թ۲쵽�������ǵ��۵⻯����Һ����ɫ��
�ʴ�Ϊ�����۵⻯����Һ����ɫ��
��4��2NaCl+2H2O=Cl2��+H2��+2NaOH
22.4L 2mol
0.0448L 0.004mol
C=
=
=0.08mol/L
�ʴ�Ϊ��0.08mol/L
��ⱥ��ʳ��ˮʱ�������ӵõ����������������ӣ�������������A���������ӵõ�������������������ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2����
������ʧ���������������������ӣ������������ϼ�B����������ʧ������������������������Ӧ���缫��ӦʽΪ��2Cl--2e-=Cl2����
�ʴ�Ϊ��2H++2e-=H2����2Cl--2e-=Cl2����
��2��A���ϲ�����������������ˮ���ռ���������Ϊ�������ܶ�С��ˮ�ģ�����Ҫ����������ˮ���ռ������̵���Ϊ�����ܣ�������Ϊ��ˮ�ܣ���������˳��ΪA��G��F����Ϊ�ռ��������������25mL������Ҫ��100mL����Ͳ�ռ�ˮ������F����H��
B���ϲ�����������������Ҫ������������ͨ�����۵⻯����Һ���飬������ǿ�����ԣ��ܺ͵⻯�ط�Ӧ���ɵ⣬�������۱���ɫ���������ܶ�С�ڵ⻯����Һ���ܶȣ����Գ�����Ϊ�����ܣ��̵���Ϊ�����ܣ������ж���ֱ���ſ���Ⱦ�������������ͼӦ���������ʣ����Կ��ü�Һ���ն������������������˳��ΪB��D��E��C����
�ʴ�Ϊ��A��G��F��H��B��D��E��C����
��3����ΪCl2+2KI=I2+2KCl���������۱���ɫ�����Թ۲쵽�������ǵ��۵⻯����Һ����ɫ��
�ʴ�Ϊ�����۵⻯����Һ����ɫ��
��4��2NaCl+2H2O=Cl2��+H2��+2NaOH
22.4L 2mol
0.0448L 0.004mol
C=
| n |
| V |
| 0.004mol |
| 0.05L |
�ʴ�Ϊ��0.08mol/L
���������⿼���˵��ԭ�����Ȿ��ʱҪע������������˳��ϴ��װ���dz����ܽ����̵��ܳ�������ˮ���ռ������Ƕ̵��ܽ��������ܳ������ж�����һ����β������װ�ã�

��ϰ��ϵ�д�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
�����Ŀ