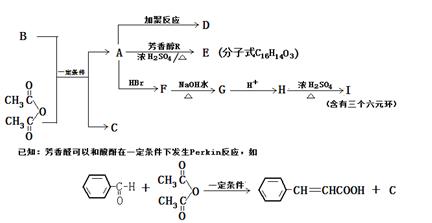
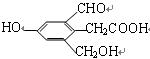
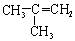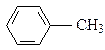��Ŀ����
(13��)�л���A��C10H20O2������������ζ����֪��
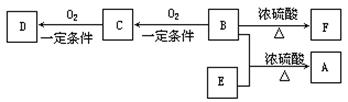
��B������û��֧����
��D����̼��������Һ��Ӧ�ų�������̼��
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�塣E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֡���F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ�� �� ��ѡ����ţ���
��ȡ����Ӧ ����ȥ��Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��D��F���������Ĺ����ŵ����������� �� �� �� ��
��3��д����D��E������ͬ����������ͬ���칹��Ľṹ��ʽ��
�� ��
��4��B��E��Ӧ����A�Ļ�ѧ��Ӧ����ʽ ��
��5��ijѧ������C�Ĺ�����ʱ��ȡ1mol/LCuSO4��Һ��2mol/LNaOH��Һ��1mL����һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL40%��C�����Ⱥ���ɫ�������֡���ͬѧʵ��ʧ�ܵ�ԭ������� �� ��ѡ����ţ�
�ټ����C���� �ڼ����C̫��
�ۼ���CuSO4��Һ�������� �ܼ���CuSO4��Һ��������

��B������û��֧����
��D����̼��������Һ��Ӧ�ų�������̼��
��D��E��Ϊ������ͬ�����ŵ�ͬ���칹�塣E���������ϵ�������Clȡ������һ�ȴ���ֻ��һ�֡���F����ʹ������Ȼ�̼��Һ��ɫ��
��1��B���Է����ķ�Ӧ�� �� ��ѡ����ţ���
��ȡ����Ӧ ����ȥ��Ӧ �ۼӾ۷�Ӧ ��������Ӧ
��2��D��F���������Ĺ����ŵ����������� �� �� �� ��
��3��д����D��E������ͬ����������ͬ���칹��Ľṹ��ʽ��
�� ��
��4��B��E��Ӧ����A�Ļ�ѧ��Ӧ����ʽ ��
��5��ijѧ������C�Ĺ�����ʱ��ȡ1mol/LCuSO4��Һ��2mol/LNaOH��Һ��1mL����һ֧�ྻ���Թ��ڻ�Ϻ��������ּ���0.5mL40%��C�����Ⱥ���ɫ�������֡���ͬѧʵ��ʧ�ܵ�ԭ������� �� ��ѡ����ţ�
�ټ����C���� �ڼ����C̫��
�ۼ���CuSO4��Һ�������� �ܼ���CuSO4��Һ��������
����15�֣�
��1���٢ڢ� �� 2 �� ��
��
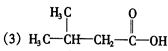
��2���Ȼ� ��2�֣���̼̼˫���� 2 �֣�
��2�֣���̼̼˫���� 2 �֣�
 �� 2 �֣�
�� 2 �֣�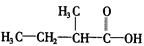 �� 2 �֣�
�� 2 �֣�
��4��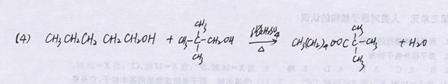 ��3 �֣� ��5���� �� 2 �֣�
��3 �֣� ��5���� �� 2 �֣�
��1���٢ڢ� �� 2 ��
 ��
����2���Ȼ�
 ��2�֣���̼̼˫���� 2 �֣�
��2�֣���̼̼˫���� 2 �֣� �� 2 �֣�
�� 2 �֣� �� 2 �֣�
�� 2 �֣���4��
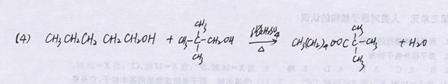 ��3 �֣� ��5���� �� 2 �֣�
��3 �֣� ��5���� �� 2 �֣���

��ϰ��ϵ�д�
�����Ŀ
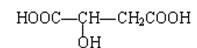
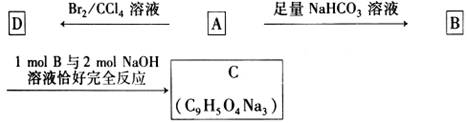
 �ɷ�Ӧ ��������Ӧ �ܼӾ۷�Ӧ ����ȥ��Ӧ
�ɷ�Ӧ ��������Ӧ �ܼӾ۷�Ӧ ����ȥ��Ӧ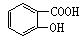 ����ش��������⣺
����ش��������⣺ ��Ϊͬ���칹�壬�����ڷ���������ȩ�����Ľṹ��ʽ(дһ�ּ���)�� ��
��Ϊͬ���칹�壬�����ڷ���������ȩ�����Ľṹ��ʽ(дһ�ּ���)�� ��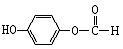 ��1mol���л������������������������ƾ���һϵ�з�Ӧ������������������Ƶ����ʵ���Ϊ������ţ� ��
��1mol���л������������������������ƾ���һϵ�з�Ӧ������������������Ƶ����ʵ���Ϊ������ţ� ��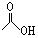 ����һ�������£�ˮ�������������ɺϳɰ�˾ƥ�֣���˾ƥ�ֿɱ�ʾΪ��
����һ�������£�ˮ�������������ɺϳɰ�˾ƥ�֣���˾ƥ�ֿɱ�ʾΪ��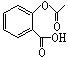 ��˾ƥ�ֵķ���ʽΪ ��
��˾ƥ�ֵķ���ʽΪ �� ��ҩƬ�Ƿ���ʵ��Լ��ǣ�ֻѡһ���Լ����ɣ�
��ҩƬ�Ƿ���ʵ��Լ��ǣ�ֻѡһ���Լ����ɣ� 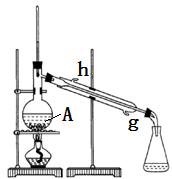
 CH3COOCH2CH2CH(CH3)2+H2O
CH3COOCH2CH2CH(CH3)2+H2O L��
L��