��Ŀ����
2��ij��ѧ����С���ú���Ϊԭ����ȡ��������ˮ������CCl4�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ����ʵ������ɷֽ�Ϊ���¼�����A����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
B����50mL��ˮ��15mL CCl4�����Һ©���У����Ǻò�������
C�������Һ©���������Ͽڲ������Ƿ�©Һ��
D����ת©������������ʱ�����������������رջ������ѷ�Һ©��������
E���ſ����������ձ�������Һ��
F���ӷ�Һ©���Ͽڵ����ϲ�ˮ��Һ��
G����©���ϿڵIJ���������ʹ��Һ�ϵİ��ۻ�С��©�����ϵ�С�ף�
H�����á��ֲ㣮
�ʹ�ʵ�飬���������գ�
��1����ȷ���������˳���ǣ��������������ı����ĸ��գ���
C��B��D��A��G��H��E��F��
��2������E����IJ�����Ӧע��ʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ��������
��3������G���������Ŀ���Dz���ʱ©����Һ���ܹ�������
��4����ѡ��CCl4�ӵ�ˮ����ȡ���ԭ����CCl4��ˮ�����ܣ����ҵ���CCl4�е��ܽ�ȱ���ˮ�еĴ�ö࣮
��5���������ʲ�����Ϊ����ˮ����ȡ����ܼ���A��
A���ƾ� B������һ���л��ܼ�����ˮ�������ܣ��ܶȱ�ˮС�� C�����Ȼ�̼��
���� ��1��CCl4�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ������Ϊ��©��װҺ�������á���Һ��
��2�����������������²�Һ�壻
��3����G�������ʹҺ��˳�����£�
��4������ˮ�����Ȼ�̼�е��ܽ��Բ�ͬ��
��5����ȡ����ѡ��ԭ����������һ����������ȡ���е��ܽ�ȱ���ˮ��Ҫ��öࣻ������ȡ����ˮ�����ܽ��ֳܷ����㣻������ȡ�������ʲ��ᷢ����Ӧ��
��� �⣺��1��CCl4�ӵ�ˮ����ȡ�Ⲣ�÷�Һ©������������Һ������Ϊ��©��װҺ�������á���Һ������ΪC��B��D��A��G��H��E��F���ʴ�Ϊ��C��B��D��H��
��2�����������������²�Һ�壬Ӧʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ���������ʴ�Ϊ��ʹ©���¶˹ܿڽ����ձ��ڱڣ���ʱ�رջ�������Ҫ���ϲ�Һ��������
��3����G������Ŀ��Ϊ����ʱ©����Һ���ܹ��������ʴ�Ϊ������ʱ©����Һ���ܹ�������
��4��ѡ��CCl4�ӵ�ˮ����ȡ���ԭ����CCl4��ˮ�����ܣ����ҵ���CCl4�е��ܽ�ȱ���ˮ�еĴ�ö࣬�ʴ�Ϊ��CCl4��ˮ�����ܣ����ҵ���CCl4�е��ܽ�ȱ���ˮ�еĴ�öࣻ
��5���ƾ���ˮ������������ܣ����ֲ㣬������Ϊ��ȡ�����ʴ�Ϊ��A��
���� ���⿼���˷�Һ�IJ������ȡ����ѡ��ѡ����ȡ��������ȡ����ѡ����жϼ��ɣ���Ŀ�Ѷ��еȣ�

| A�� | ${\;}_{92}^{235}$U���ԭ������Ϊ235 | |
| B�� | ${\;}_{92}^{235}$U�е�������Ϊ92 | |
| C�� | ${\;}_{92}^{235}$U�� ${\;}_{6}^{12}$C��������ԼΪ235��12 | |
| D�� | ${\;}_{92}^{235}$U ��${\;}_{92}^{238}$U����Ϊͬλ�� |
| A�� | ʵ�����ϵľƾ����㵹��ȼ��������������ˮ���� | |
| B�� | ����������Һ�������ڣ����������۾���������� | |
| C�� | Ƥ���Ͻ��н϶��Ũ���ᣬ�Ͻ���ˮ��ϴ | |
| D�� | ��Һʱ���²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| A�� | pH=2�Ĵ�����Һ��pH=12������������Һ�������ϣ���Ϻ���ҺpH=7 | |
| B�� | ��0.1mol•L-1����������Һ��pH=1ϡ����������ϣ���Ϻ����ҺpH��7 | |
| C�� | �������Һ�У�c��SO42-����c��NH4+����c��H+����c��OH-�� | |
| D�� | pH��ͬ�Ĵ�������Һ��̼��������Һ��̼������Һ�������ʵ���Ũ�ȣ� c��CH3COONa����c��NaHCO3����c��Na2CO3�� |
| Ԫ�� | �����Ϣ |
| X | XԪ�ؿ��γ���Ȼ����Ӳ���������� |
| Y | �䵥��Ϊ˫ԭ�ӷ��ӣ�������⻯���ˮ��Һ��ʹ��̪��� |
| Z | Z�Ƕ�������������ʧȥ���ӵ�Ԫ�� |
| M | M��һ��ͬλ�ص�������Ϊ34��������Ϊ18 |
| N | N�Ǿ����Ϻ�ɫ����Ľ������кܺõ���չ�ԡ������Ժ͵����� |
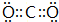 ��
����2����״���£���4.48L X�����������ͨ��1L 0.3mol•L-1 Z������������Ӧˮ�������Һ�з�����Ӧ�����ӷ���ʽΪ3OH-+2CO2=HCO3-+CO32-+H2O��
��3��Y���⻯��Y2H4�ǻ���ij���ȼ�ϣ���֪16.0g��Һ̬ȼ������������ȫȼ�����ɵ�����Һ̬ˮʱ���ų�312kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624 kJ•mol-1��
��4��Ԫ��N������������е������Լ�������Ӧ�����ɳ����ᣨHO2����N+HCl+O2�TNCl+HO2��HO2������һ���������Ҳ��һ�����ɻ������м��ߵĻ��ԣ������йظ÷�Ӧ��˵���������C��
A����������O2
B��HO2�ڼ��в����ȶ�����
C������������HO2
D.1mol N�μӷ�Ӧ����1mol���ӷ���ת��
��5��Y��Z��Ԫ���γɵĻ�����H�����������İ�ȫ���ң�ȡ6.5g H���ײ���ֽ��õ����ֵ��ʣ�����һ�����嵥�ʵ�����ڱ�״����Ϊ3.36L����H�Ļ�ѧʽΪNaN3�������ʵľ�������Ϊ���Ӿ��壮
 A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺
A��B��C��D��Ԫ�����ڱ���ǰ36��Ԫ�أ����ǵĺ˵�����������ڶ�����Ԫ��Aԭ�ӵĺ���ɶԵ�������δ�ɶԵ�������2������3���ܼ���Bԭ�ӵ������p����ĵ���Ϊ������ṹ��C�ǵؿ��к�������Ԫ�أ�D�ǵ�������Ԫ�أ���ԭ�Ӻ�����������������ԭ����ͬ�����������Ӿ���������ش��������⣺ ��ͼ��ʾ��������A��װ��20���ˮ50mL������B��װ��1 mol/L������50mL���Թ�C��D����ͨ��������װ�к���ɫNO2����ɫN2O4�Ļ�����壬����������ƽ�⣺
��ͼ��ʾ��������A��װ��20���ˮ50mL������B��װ��1 mol/L������50mL���Թ�C��D����ͨ��������װ�к���ɫNO2����ɫN2O4�Ļ�����壬����������ƽ�⣺