��Ŀ����
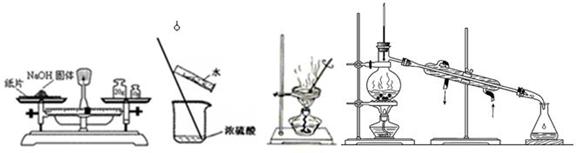
��14�֣�ʵ����������0.5 mol/L��NaOH��Һ500 ml���������������ձ�����100 ml��Ͳ����100 ml����ƿ����500 ml����ƿ���ݲ���������������ƽ(������)��
��1������ʱ������ʹ�õ������� ������ţ�����ȱ�ٵ������� ���������������õ��������������÷ֱ��� �� ��
��2��ʹ������ƿǰ������е�һ�������� ��
��3��������Һʱ��һ����Է�Ϊ���¼������裺�ٳ������ڼ��㣻���ܽ⣻��ҡ�ȣ���ת�ƣ���ϴ�ӣ��߶��ݣ�����ȴ������ȷ�IJ���˳��Ϊ ��
��4�������ƹ�����������������ȷ�ģ����в������������ƫ�ߵ��� ��
��û��ϴ���ձ��Ͳ����� ��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ�������
������ƿ�����������������ˮ�ܶ���ʱ���ӱ��� �ݶ���ʱ���ӱ���
��1������ʱ������ʹ�õ������� ������ţ�����ȱ�ٵ������� ���������������õ��������������÷ֱ��� �� ��
��2��ʹ������ƿǰ������е�һ�������� ��
��3��������Һʱ��һ����Է�Ϊ���¼������裺�ٳ������ڼ��㣻���ܽ⣻��ҡ�ȣ���ת�ƣ���ϴ�ӣ��߶��ݣ�����ȴ������ȷ�IJ���˳��Ϊ ��
��4�������ƹ�����������������ȷ�ģ����в������������ƫ�ߵ��� ��
��û��ϴ���ձ��Ͳ����� ��δ��NaOH��Һ��ȴ�����¾�ת�Ƶ�������
������ƿ�����������������ˮ�ܶ���ʱ���ӱ��� �ݶ���ʱ���ӱ���
��1���٢ܢݢޣ� ��ͷ�ιܡ������ܽ⣬���� ��2������Ƿ�©Һ
��3���ڢ٢ۢ�ݢޢߢ� ��4���ڢ�
��3���ڢ٢ۢ�ݢޢߢ� ��4���ڢ�
��1��������Ҫ������������ȡ���ܽ���Ҫ�ձ��Ͳ�������������Ҫ��Ӧ��������ƿ��������Ӧ��ͷ�ιܣ�������Ҫ�������Ǣ٢ܢݢޣ���ȱ�ٽ�ͷ�ιܣ��ܽ�ʱͨ���������Ľ�������ܽ⣬ת��Һ��ʱͨ����������������������
��2��ʹ������ƿ֮ǰ�����������ƿ�Ƿ�©Һ��
��3���������Ƶ�ԭ����ʵ����̿�֪����ȷ�IJ���˳���Ǣڢ٢ۢ�ݢޢߢܡ�
��4������c��n/V��֪�����û��ϴ���ձ��Ͳ������������ʼ��٣�Ũ��ƫ�ͣ�δ��ȴ������ȴ������ƿ����Һ��������٣�����Ũ��ƫ�ߣ�����ƿ�����������������ˮ�����ʺ���Һ������仯��Ũ�Ȳ��䣻����ʱ���ӱ��ߣ�������ƿ����Һ��������٣�Ũ��ƫ�ߣ���֮���ӣ�Ũ��ƫ�ͣ���ѡ�ڢܡ�
��2��ʹ������ƿ֮ǰ�����������ƿ�Ƿ�©Һ��
��3���������Ƶ�ԭ����ʵ����̿�֪����ȷ�IJ���˳���Ǣڢ٢ۢ�ݢޢߢܡ�
��4������c��n/V��֪�����û��ϴ���ձ��Ͳ������������ʼ��٣�Ũ��ƫ�ͣ�δ��ȴ������ȴ������ƿ����Һ��������٣�����Ũ��ƫ�ߣ�����ƿ�����������������ˮ�����ʺ���Һ������仯��Ũ�Ȳ��䣻����ʱ���ӱ��ߣ�������ƿ����Һ��������٣�Ũ��ƫ�ߣ���֮���ӣ�Ũ��ƫ�ͣ���ѡ�ڢܡ�

��ϰ��ϵ�д�
�����Ŀ
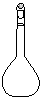 ����������
���������� ����������
����������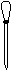 ����������
����������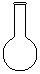 ����������
����������