��Ŀ����
H2C2O4Ϊ��Ԫ���ᡣ20������һ��c(H2C2O4)+c(HC2O4�C)+c(C2O42�C)=0.100 mol��L�C1��H2C2O4��NaOH�����Һ����Һ�в����������ʵ���Ũ����pH�ı仯������ͼ��ʾ��һ����ȷ����
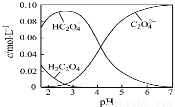
A��pH=2.5����Һ�У�c(H2C2O4)+c(C2O42�C)��c(HC2O4�C)
B��c(Na+)=0.100 mol��L�C1����Һ�У�c(H+)+c(H2C2O4)=c(OH�C)+c(C2O42�C)
C��c(HC2O4�C)=c(C2O42�C)����Һ�У�c(Na+)��0.100 mol��L�C1+c(HC 2O4�C)
2O4�C)
D��pH=7����Һ�У�c(Na+)<2c(C2O42�C)
 �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д���1 L��0.01 mol NaAlO2��0.02 mol NaOH����Һ�л���ͨ��CO2����n(CO2)�����Ⱥ���������ͬ�ķ�Ӧ����0.01 mol<n(CO2)  0.015ʱ�����ķ�Ӧ�ǣ�2NaAlO2+ CO2+3H2O=2Al(OH)3��+Na2CO3�����ж�Ӧ��ϵ��ȷ����
0.015ʱ�����ķ�Ӧ�ǣ�2NaAlO2+ CO2+3H2O=2Al(OH)3��+Na2CO3�����ж�Ӧ��ϵ��ȷ����
ѡ�� | n(CO2)/mol | ��Һ�����ӵ����ʵ���Ũ�� |
A | 0 | c(Na+)>c(AlO2-)+c(OH-) |
B | 0.01 | c(Na+)>c(AlO2-)>c(OH-)>c(CO32-) |
C | 0.015 | c(Na+)>c(HCO3-)>c(CO32-)> c(OH-) |
D | 0.03 | c(Na+)>c(HCO3-)> c(OH-)>c(H+) |
��ѧ��Ӧ������ͨ��ʵ��ⶨ�ģ����л�ѧ��Ӧ���ʵIJ����У��������ݲ� ��
�� ���( )
���( )
ѡ�� | ��ѧ��Ӧ | ��������(��λʱ����) |
A | 2NO2 | ��ɫ��dz |
B | Zn+H2SO4=ZnSO4+H2 | H2��� |
C | CO(g)+H2O(g)=CO2(g)+H2(g) | ѹǿ�仯 |
D | Ca(OH)2+Na2CO3=CaCO3��+2NaOH | �������� |


 CH3CH2OH+CH3COOH
CH3CH2OH+CH3COOH
 42��)+c(CH3COO��)+c(OH��)
42��)+c(CH3COO��)+c(OH��)
 N2O4
N2O4