��Ŀ����
��18�֣���A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������B��C��D�������ӡ�A�������Ӿ���������ԭ����ͬ�ĵ��Ӳ�ṹ��A��B���γ����ӻ�����B2A��D����������������ǿ�ᷴӦ��������ǿ�Ӧ��E��ԭ�ӽṹʾ��ͼΪ��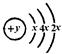 ��F�������������ǵ��Ӳ�����2����
��F�������������ǵ��Ӳ�����2����
�Իش����и����⣺
��1��BԪ��Ϊ ����Ԫ�ط��ţ�
��2��FԪ��λ��Ԫ�����ڱ��е� ���ڵ� ��
��3��������B2A�ĵ���ʽ____________________
��4��D�������������B������������Ӧ��ˮ������Һ��Ӧ�����ӷ���ʽ��
��5����C��D�õ�����������B������������Ӧ��ˮ�������Һ�У��γ�ԭ��أ������ĵ缫��ӦΪ
 ��F�������������ǵ��Ӳ�����2����
��F�������������ǵ��Ӳ�����2�����Իش����и����⣺
��1��BԪ��Ϊ ����Ԫ�ط��ţ�
��2��FԪ��λ��Ԫ�����ڱ��е� ���ڵ� ��
��3��������B2A�ĵ���ʽ____________________
��4��D�������������B������������Ӧ��ˮ������Һ��Ӧ�����ӷ���ʽ��
��5����C��D�õ�����������B������������Ӧ��ˮ�������Һ�У��γ�ԭ��أ������ĵ缫��ӦΪ
��1��Na ��2��3����A ��3��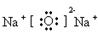 ��4��Al2O3+2OH-=2AlO2-+H2O
��4��Al2O3+2OH-=2AlO2-+H2O
��5��2H2O-2e-=H2��+2OH- ��2H++2e-=H2�� ��ÿ��3�֣���18�֣�
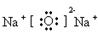 ��4��Al2O3+2OH-=2AlO2-+H2O
��4��Al2O3+2OH-=2AlO2-+H2O ��5��2H2O-2e-=H2��+2OH- ��2H++2e-=H2�� ��ÿ��3�֣���18�֣�
��

��ϰ��ϵ�д�
 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�
�����Ŀ

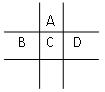
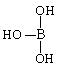 ������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H������д����������ˮ����Һ�����Ե����ӷ���ʽ�� ����4�֣�
������������ˮ��1�����������ˮ���ã�ֻ�ܲ���1��H������д����������ˮ����Һ�����Ե����ӷ���ʽ�� ����4�֣�