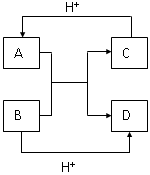
��Ŀ����
��12�֣���A��B��C��D��E���ֶ�����Ԫ�أ���֪���ڵ�A�� B��C��D����Ԫ��ԭ�Ӻ����56�����ӣ������ڱ��е�λ����ͼ��ʾ��E�ĵ��ʿ����ᷴӦ��1molE���������������ã��ڱ�״�����ܲ���33.6LH2��E����������A�������Ӻ�����Ӳ�ṹ��ȫ��ͬ���ش��������⣺
��1������Ԫ�ص����ƣ�A______��B______�� C______��D______��E______��
��2��B��C��DԪ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ__________________________��
���ǵ�����������Ӧˮ���������ǿ��˳��Ϊ_________________________��
��3��д��C����������A������ȼ�պ�IJ�����D����ͬʱͨ��ˮ�еĻ�ѧ����ʽ ____��
��4��A��E�γɵĻ�����Ļ�ѧʽ�� ____���������ʾ������������ʵķ�����Ӧ���� ____��
��5����D��E�γɵĻ������ˮ��Һ�е����ռ���Һֱ������ʱ���۲쵽�������� ____���йط�Ӧ�����ӷ���ʽΪ ____��

��1������Ԫ�ص����ƣ�A______��B______�� C______��D______��E______��
��2��B��C��DԪ�ص�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ__________________________��
���ǵ�����������Ӧˮ���������ǿ��˳��Ϊ_________________________��
��3��д��C����������A������ȼ�պ�IJ�����D����ͬʱͨ��ˮ�еĻ�ѧ����ʽ ____��
��4��A��E�γɵĻ�����Ļ�ѧʽ�� ____���������ʾ������������ʵķ�����Ӧ���� ____��
��5����D��E�γɵĻ������ˮ��Һ�е����ռ���Һֱ������ʱ���۲쵽�������� ____���йط�Ӧ�����ӷ���ʽΪ ____��
(ÿ��1�֣���12��)
(1) �� �� �� �� ��
��2��r(P) > r (S) > r (Cl) HClO4>H2SO4>H3PO4
��3��SO2 + Cl2 + 2H2O="=" H2SO4 + 2HCl
��4��Al2O3 ����������
��5���а�ɫ�������ɣ����ﵽ�����ʱ�����٣����ճ�����ȫ��ʧ����ɳ�����Һ��
Al3+ + 3OH-= Al(OH)3 �� Al(OH)3 + OH-=AlO2- + 2H2O
(1) �� �� �� �� ��
��2��r(P) > r (S) > r (Cl) HClO4>H2SO4>H3PO4
��3��SO2 + Cl2 + 2H2O="=" H2SO4 + 2HCl
��4��Al2O3 ����������
��5���а�ɫ�������ɣ����ﵽ�����ʱ�����٣����ճ�����ȫ��ʧ����ɳ�����Һ��
Al3+ + 3OH-= Al(OH)3 �� Al(OH)3 + OH-=AlO2- + 2H2O
��

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
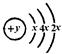 ��F�������������ǵ��Ӳ�����2����
��F�������������ǵ��Ӳ�����2���� ��Ϊ �����γɻ�����ʱ��������ϼ�Ϊ ��
��Ϊ �����γɻ�����ʱ��������ϼ�Ϊ ��